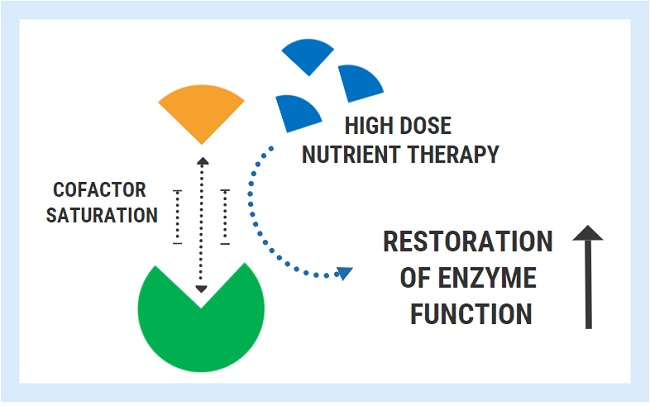
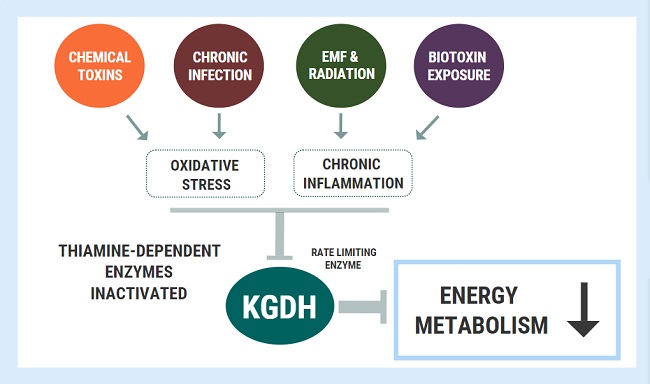
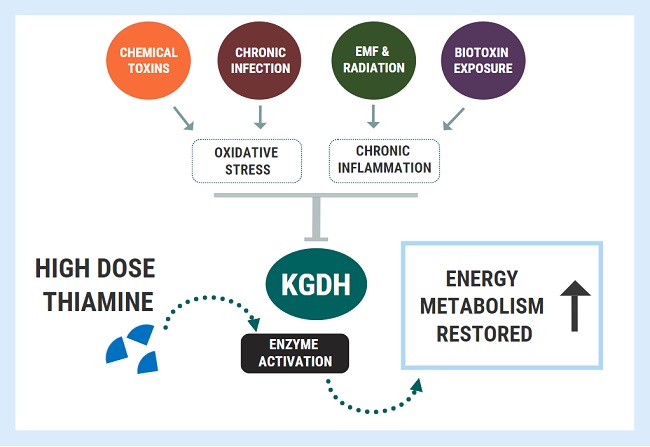
Diagnostic overshadowing is a phrase used to describe a cognitive bias employed by many practitioners. It assumes that all of a patient’s symptoms can be ascribed to a particular pre-existing or chronic condition. This is common in pediatrics, where health issues in children with complex needs, such as Down syndrome, are misattributed to the Down syndrome and not investigated or addressed independently. This leads to delayed diagnoses and treatment, and in many cases, poorer outcomes. It is also common when the root of the ill-health emerges from vitamin deficiencies. By way of example and with the parent’s permission, below is the case of a two-year old boy who developed both wet and dry beriberi due to thiamine deficiency. His condition was worsened by medical treatments and missed because of diagnostic overshadowing.
When Real Treatable Conditions Are Missed
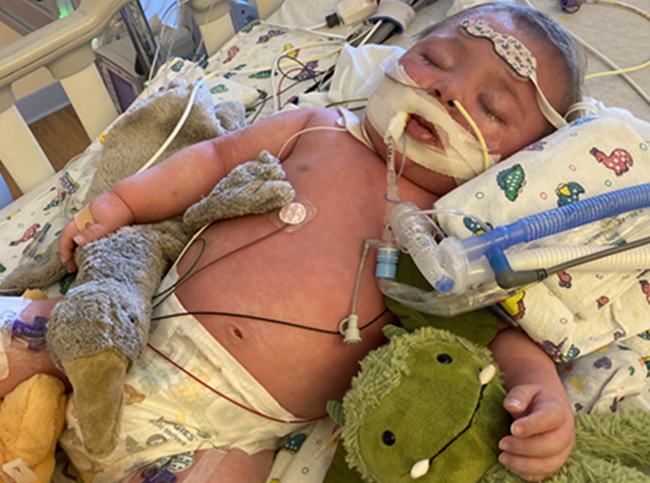
Lev is a bright-eyed, curious two-year-old with Down syndrome. His eyes light up when he hears familiar voices, and he delights in interacting with his parents and siblings. Behind his bright smile, however, lies a complicated medical journey. Like many children with complex medical needs, his early years have been filled with specialist visits, medications, and hospitalizations. For much of his short life, he has been profoundly weak, struggling to gain weight, battling constant vomiting and diarrhea, and falling far behind in gross motor development. What Lev’s story illustrates most powerfully is the danger of diagnostic overshadowing: when real, treatable conditions are missed simply because a child has a known genetic diagnosis.
Lev’s story is complex. Born at 39 weeks with congenital heart defects (a large VSD and ASD), intrauterine growth restriction, and early respiratory distress, he spent his first 13 months in the hospital. By three months old he developed seizures, and by six months he was diagnosed with pulmonary hypertension. He required a GJ-feeding tube, a tracheostomy tube, and was given multiple cardiovascular medications, including high-dose Lasix (furosemide), a loop diuretic known to deplete thiamine (vitamin B1).[1],[2],[3],[4] Despite the intensity of his medical care and frequent hospitalizations, his worsening weakness and developmental regression were never investigated beyond his genetic diagnosis. His inability to lift his head or bear weight was simply attributed to “Down syndrome,” and his declining function was accepted as inevitable. No one on his conventional medical team ever evaluated him for B1 deficiency.
At two years old, Lev has not yet undergone the life-saving surgery to repair his VSD and ASD, an intervention that many children with Down syndrome receive in infancy, because his profound weakness, frequent infections, and uncontrolled pulmonary hypertension have made him too medically fragile to tolerate the procedure.
His mother, worried about his persistent vomiting, diarrhea, poor tone, and developmental delays, began researching on her own. When she came across the symptoms of pediatric beriberi, the severe form of thiamine deficiency, she brought it to the attention of his doctors. They dismissed her concerns.
Fortunately, she persisted.
Profound Mitochondrial Dysfunction
She brought Lev to me after watching my online lecture “Thiamine Deficiency in Children with Special Needs”. At our first visit, it was clear that Lev was experiencing profound mitochondrial dysfunction. He was being fed via GJ-tube with a formula that didn’t provide adequate thiamine to meet his needs. He had been exposed to more than 10 rounds of antibiotics for pneumonia, which likely disrupted his gut flora and impaired his nutrient absorption. He was still taking Lasix, a medication known to deplete thiamine, yet no one had evaluated his thiamine status.
My initial recommendations without any testing included:
- TTFD (thiamine tetrahydrofurfuryl disulfide) – 50 mg daily in the morning
- Riboflavin 5-phosphate – 25 mg daily in the morning
- Magnesium glycinate – 60 mg daily throughout the day
- Polyenylphosphatidylcholine – 900 mg daily in the morning
- Vitamin D – 800 IU daily anytime of day
- Iron bisglycinate – 12 mg daily, preferably on an empty stomach
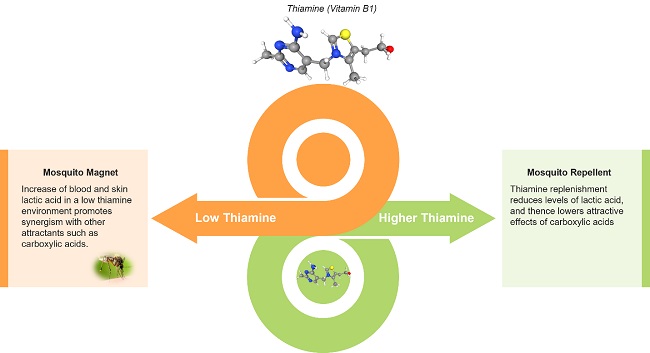
Lev’s story is not unique in my practice. I’ve identified thiamine deficiency in many children with Down syndrome, often after months or even years of unexplained symptoms that were overlooked or misattributed. Children with Down syndrome are especially vulnerable to thiamine deficiency due to slower gastrointestinal motility, which increases the risk of small intestinal bacterial overgrowth (SIBO) and subsequent nutrient malabsorption.[5] Unfortunately, these underlying contributors are rarely acknowledged in conventional care. Nearly all of my patients have experienced some form of diagnostic overshadowing, where serious but treatable issues are dismissed as “just part of Down syndrome.” This pattern is far too common and far too harmful.
We proceeded with further testing, including an organic acid test and microbial stool analysis, to better understand the underlying contributors to his complex symptoms.
A Two Year Old With Wet and Dry Beriberi
When Lev’s lab results returned, they were staggering. His organic acid test showed:
- Severely elevated pyruvic acid, lactic acid, and alpha-keto acids – textbook markers of pyruvate dehydrogenase dysfunction, a hallmark of B1 deficiency
- Broad mitochondrial failure, with elevated markers across the entire Krebs cycle
- Elevated tartaric acid and D-arabinitol, suggesting significant Candida overgrowth
- Functional markers of B12, folate, B6, CoQ10, and magnesium deficiencies
- Elevated quinolinic acid, indicating neuroinflammation
- Oxidative stress with high lipid peroxides and 8-OHdG
His stool test revealed a severely imbalanced microbiome:
- Overgrowth of Enterobacter cloacae and Candida albicans
- Absence of Lactobacillus and E. coli, both important for nutrient absorption and gut health
- Overgrowth of Clostridium species, which may contribute to inflammation and further disrupt digestion
The conclusion was clear: Lev was suffering from wet and dry beriberi, driven by severe thiamine deficiency, worsened by chronic diuretic use and malabsorption. His seizures, vomiting, poor tone, delayed gross motor skills, and even pulmonary hypertension could all be traced back to a lack of essential B vitamins, especially thiamine. [6],[7], [8]
By the time he came to my clinic, Lev could not even lift his head when placed on his belly, a basic milestone typically achieved in the first months of life. His early seizures (including infantile spasms) had resolved with medication, but their cause had never been identified. In hindsight, these seizures were likely driven by energy failure in the brain, a known consequence of B1 and other B vitamin deficiencies that impair mitochondrial function and neurotransmitter balance.[9]
Within days of starting thiamine and other supports, his mother noticed small but encouraging changes: Lev became more alert, more interactive, and began reaching for toys for the first time, as well as holding his head up when prone (on his belly). His vomiting and reflux diminished. His digestion improved. His body, for the first time in a long time, was beginning to catch up.
My recommendations after reviewing his lab results and discussing them thoroughly with his parents included:
- Nystatin 500,000 unit tablets – ½ tablet 4 times per day
- Biocidin – 2 drops twice a day, increasing dose slowly over one week
- Lactobacillus rhamnosus GG – 15 billion per day, given away from Biocidin
- TTFD – 200 mg per day in the morning
- Liposomal CoQ10 – 125 mg per day
- L-carnitine – 635 mg per day
- Active B Complex – 1 capsule per day
- Thiamin (hydrochloride, benfotiamine): 30 mg
- Riboflavin (riboflavin-5-phosphate): 10 mg
- Niacin (inositol hexaniacinate): 100 mg
- Vitamin B6 (pyridoxal-5-phosphate): 25 mg
- Folate (from (6S)-5-methyltetrahydrofolic acid [MTHF], glucosamine salt, Quatrefolic®): 680 mcg DFE
- Vitamin B12 (methylcobalamin): 500 mcg
- Biotin: 250 mcg
- Pantothenic Acid (calcium D-pantothenate): 100 mg
- Choline (dihydrogen citrate): 50 mg
- Inositol: 25 mg
- R-alpha lipoic acid – 50 mg per day
- Potassium citrate – 224 mg per day
- Continue:
- Riboflavin 25 mg per day
- Magnesium glycinate 60 mg per day
- Polyenylphosphatidylcholine 900 mg daily in the morning
- Vitamin D 800 IU daily anytime of day
- Iron bisglycinate 12 mg daily, preferably on an empty stomach
The Bigger Picture: Diagnostic Overshadowing in Down Syndrome
Lev’s story is a powerful and heartbreaking example of diagnostic overshadowing, a common but often unspoken problem in the care of children with Down syndrome. This occurs when medical professionals attribute new, worsening, or unexplained symptoms to the child’s known diagnosis rather than investigating further. In Lev’s case, his profound weakness, inability to lift his head, chronic vomiting, diarrhea, and history of seizures were all seen as “typical for Down syndrome.” But they weren’t. They were red flags for severe nutrient deficiencies, particularly thiamine (vitamin B1).
It is imperative for physicians, especially specialists working in critical care units, to recognize the profound impact that vitamins and vitamin deficiencies can have on the physiology of their pediatric patients. In children with complex medical conditions, underlying micronutrient imbalances often go undetected, yet they can significantly impair mitochondrial function, immune regulation, neurological development, and cardiovascular stability. Medications commonly used in hospital settings, such as diuretics, antiepileptics, and proton pump inhibitors, can further deplete essential nutrients like thiamine, magnesium, and B12, compounding the medical vulnerability of these children. A deeper understanding of nutritional biochemistry is essential for preventing avoidable deterioration, improving outcomes, and delivering truly comprehensive pediatric care.
In children with Down syndrome, symptoms like poor muscle tone, delayed milestones, constipation or diarrhea, fatigue, and even seizures are frequently dismissed as part of the condition. This mindset can be deeply harmful. When clinicians stop asking why a symptom is happening, especially when that symptom is new or worsening, they miss opportunities to identify treatable, reversible causes that can dramatically change the trajectory of a child’s health and development.
Lev’s case is sadly not unique. Thiamine deficiency is well-documented in children who are on diuretics like Lasix, who have gut dysfunction, high metabolic demands, or malabsorption – all common features in children with Down syndrome. Yet this critical nutrient is rarely tested, and even less frequently treated. In functional medicine, we are trained to look beneath the surface, to question assumptions, and to search for root causes. For Lev, the cause was clear: his thiamine was being depleted faster than it could be replenished, and no one had been monitoring this vital nutrient, until it was nearly too late.
When diagnostic overshadowing leads to inaction, children suffer unnecessarily. Lev’s story is a call to parents, caregivers, and clinicians to keep asking questions and to never assume that something is “just part of the diagnosis” without first considering what else might be going on.
Lev’s journey is not over, but he is now on a path of healing. His mother continues to advocate fiercely for his care. His treatment plan includes thiamine, mitochondrial support, targeted antimicrobial therapy, and continued nutritional repletion. His case may be complex, but it is not hopeless. He will be monitored closely under my care using functional testing to guide next steps and track progress. I hope his conventional medical team takes the time to carefully review the detailed letter I sent, which outlines the root causes we are addressing and the importance of collaborative support.
Parents – Trust Your Instincts
If you’re a parent of a child with Down syndrome, or any child with complex medical needs, trust your instincts. If something feels off, don’t stop asking questions. If you’ve ever been told, “It’s just part of the condition,” I urge you to ask again. Ask why. Ask what else could be going on. Don’t be afraid to bring up what you’ve read or researched. You know your child best, and your intuition is often the first and most reliable clue that something important is being missed.
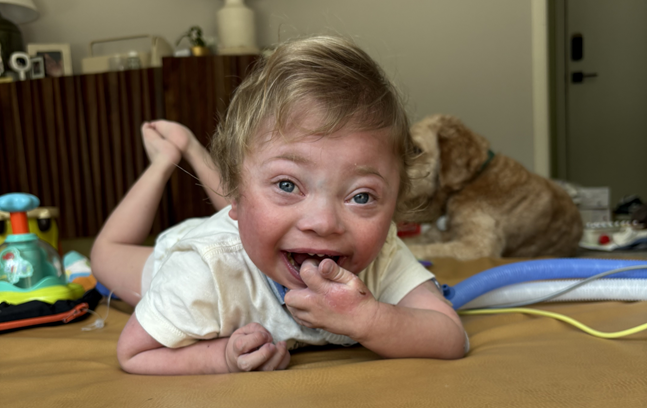
Lev’s story is proof of that. His mother recognized something deeper was going on when his professional medical team didn’t. Her persistence is what led her to me and our discovery of a severe, life-altering thiamine deficiency, a diagnosis that had been overlooked despite months of symptoms, hospitalizations, and medications. Her advocacy quite literally changed the course of his life.
If you’re a medical provider, please remember this: Down syndrome is not a catch-all explanation. It is not a reason to stop investigating. Children with Down syndrome deserve the same level of curiosity, biochemical inquiry, and individualized care as every other child. In fact, they often need it more. Micronutrient deficiencies like thiamine (B1) are easy to miss, but they are crucial to mitochondrial function, GI motility, neurodevelopment, and vascular tone. These are not minor contributors; they are foundational to a child’s health and development.
Lev’s weakness, seizures, vomiting, and severe delays were not “just part of Down syndrome.” They were symptoms of a preventable, diagnosable, and treatable condition, and tragically, they were ignored for far too long.
Let’s do better. Let’s listen closer. Let’s not miss it again.
References
[1] Rieck J, Halkin H, Almog S, Seligman H, Lubetsky A, Olchovsky D, Ezra D. Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers. J Lab Clin Med. 1999 Sep;134(3):238-43. doi: 10.1016/s0022-2143(99)90203-2.
[2] Sica DA. Loop diuretic therapy, thiamine balance, and heart failure. Congest Heart Fail. 2007 Jul-Aug;13(4):244-7. doi: 10.1111/j.1527-5299.2007.06260.x.
[3] Ritorto G, Ussia S, Mollace R, Serra M, Tavernese A, Palma E, Muscoli C, Mollace V, Macrì R. The Pivotal Role of Thiamine Supplementation in Counteracting Cardiometabolic Dysfunctions Associated with Thiamine Deficiency. Int J Mol Sci. 2025 Mar 27;26(7):3090. doi: 10.3390/ijms26073090.
[4] Ryan MP. Diuretics and potassium/magnesium depletion. Directions for treatment. Am J Med. 1987 Mar 20;82(3A):38-47. doi: 10.1016/0002-9343(87)90131-8.
[5] DiBaise JK. Nutritional consequences of small intestinal bacterial overgrowth. Pract Gastroenterol. 2008;32(12):15–28 (https://www.peirsoncenter.com/uploads/6/0/5/5/6055321/sibo_artikel.pdf)
[6] Pache-Wannaz L, Voicu C, Boillat L, Sekarski N. Case Report: severe pulmonary hypertension in a child with micronutrient deficiency. Front Pediatr. 2025 Jan 31;13:1478889. doi: 10.3389/fped.2025.1478889.
[7] C S, Kundana PK, Reddy N, Reddy B S, Poddutoor P, Rizwan A, Konanki R. Thiamine-responsive, life-threatening, pulmonary hypertensive crisis with encephalopathy in young infants: A case series. Eur J Paediatr Neurol. 2022 Jan;36:93-98. doi: 10.1016/j.ejpn.2021.12.010.
[8] Rabinowitz SS. Pediatric beriberi clinical presentation: history, physical, causes. Medscape. Updated March 17, 2014. (https://www.peirsoncenter.com/uploads/6/0/5/5/6055321/pediatric_beriberi_clinical_presentation__history_physical_causes.pdf)
[9] Lanska DJ, Fatal-Valevski A. Epilepsy in children with infantile thiamine deficiency. Neurology. 2010 Feb 23;74(8):702-3; author reply 703. doi: 10.1212/WNL.0b013e3181d2b857.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image created using Canva AI.