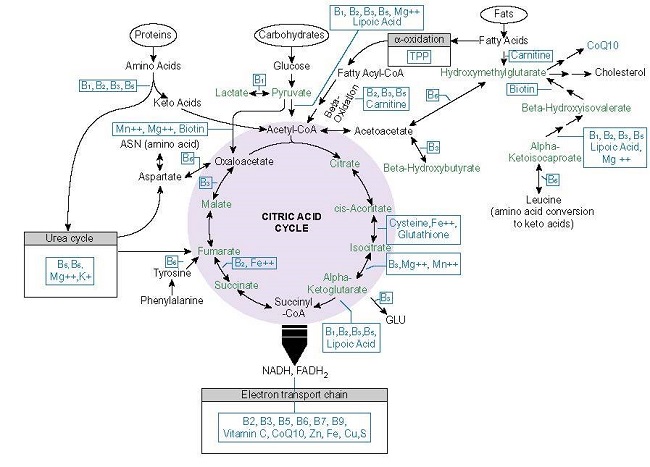
I am a 60 year old female who has been experiencing severe depression with anhedonia for over a decade. I often feel oxygen and energy deprivation in my head more often when I lie down. I have a lot of short term memory problems and executive functioning issues that began around age 50. Sometimes I feel the earth move under my feet and I am occasionally dizzy and have double vision. If I look intently at things, it appears as though they are moving. I also have some visual tracking issues. This is partly do being blind focally in one eye and floaters in both, but I suspect there is more too it. I have endured restless leg syndrome for years, which has been significantly less for the last few weeks after beginning thiamine, as have some of my other symptoms, but the depression, anhedonia and general loss of motivation and lack of joy remains. I have begun using a variety of supplements but feel as though I am still missing something. I am sharing my story in the hopes that someone can offer some help.
Childhood Through Early Adulthood
Since childhood, I have felt physically crappy. I was never able to breathe through my nose. I had asthma and constant, intense itching in my ears, nose, throat, head, and eyes. Insomnia plagued me as a child due to anxiety, along with the inability to breathe and the intense itching in my head. Regularly, and especially at night, I fantasized of putting an icepick into my ear to scratch the horrible itch in the center of my head. All day, everyday, I choked on the constant snot that continuously poured out of my nose and clogged my throat. I choked often on my food being a total mouth breather. I needed a box of Kleenex’s to get through a day. Despite it constantly running, I could not breathe through my nose at all. Encumbering as all this was, I still managed to feel somewhat hopeful, played outdoors, had friends, and attended school most days.
I chose to leave home quite young (at 15 years old) because of family dysfunction. By 16, I stopped consuming liquid dairy, thus leading to a nose-breathing liberation. I still was plagued with sinus issues but could breathe occasionally through my nose to some degree for the first time ever.
As a youth, I experimented with drugs, but never really took anything regularly as the hangovers were horrible and weakening for me. I did a fair amount of drinking in twenties as well but paid the price health wise, and since have not had a drink in many years.
In my twenties, I became aware of sugar causing severe hypoglycemia in me, caused huge mood swings and vision loss. I also self-diagnosed myself with hypothyroidism. I went to see doctors assuming this was causing my miscarriages but the doctors invalidated me at every turn, insisting I was fine. So my Hashimoto’s went untreated for many years until I discovered I could treat it with over-the-counter desiccated thyroid.
Even with all of this going on, I just kept dragging myself along through life on what felt like sheer willpower alone. During this time (my 20’s), I ate more vegetables (fresh organic) and less meat, I had a lot of stomach pain that plagued me on top of everything else, even though my diet was quite good and full of organic vegetables grown nearby. I wasn’t a trying to be a vegetarian, I always thought of myself a bit more of a carnivore, but being that I lived among vegetarians I didn’t eat meat on a daily basis. I noticed that when I did eat meat, I felt a little better. I wish I had taken it more seriously then, but I was still in my optimistic youth, and every day was a new day where I thought I was going to magically feel better.
Lifelong Anxiety and Stage Fright
Prior to the depression, I was a violinist, but one who suffered from lifelong, crippling stage fright. As a child I couldn’t sleep at all for days prior to an audition or performance, which was often. This continued my whole life. Nevertheless, I was able to push through and have performed and recorded many pieces with many different people through the years. Over time though, I began to avoid auditions, and mostly, only performed solo for strangers like at weddings and parties where there weren’t high expectations. Many times, I convinced myself to get over this anxiety, I just had to do it, to get out there and perform. This never worked. I never got over it. Oddly enough, no one realized what I was going through while I played.
I took immediate release Adderall 40-60mg 2-4 x a week for about 5 years in my late 40s to early 50s. It was prescribed for ADHD and for stage fright during violin performances. It also helped with motivation. I have always had a pretty scattered ADHD type personality and felt that I was a high functioning autistic person.
I take trazodone to help sleep when I can afford to get it, but it doesn’t always work. So lately I have been taking a break. Sometimes I will take an over the counter antihistamine/cold medicine like Tylenol when I am desperate to sleep, like when I’m caring for mother. It is a last resort though. I prefer not take anything being it makes me a little nauseous and I worry about liver damage.
I tried Wellbutrin for depression for several months about a year and a half ago, but felt nothing. I tried Prozac for four weeks in my 40s and also felt nothing.
Mumps and Loss of Vision in One Eye
I got the mumps in my forties. This was the closest I ever felt to death in my life. I subsequently lost vision in my right eye. When I lost my vision, it was assumed that I had ocular histoplasmosis but a few years prior to that I had lost vision in one eye for a few months to an unusual eye condition called MEWDS, (multiple evanescent white dot syndrome). MEWDS can be induced by a virus, perhaps having the mumps virus had something to do with it. I also wonder if I was actually type 2 diabetic off and on in my life, or at least borderline, and if that cost me my eye.
Debilitating Depression
After a lifetime of feeling crappy, multiple miscarriages, carpal tunnel, loss of vision in one eye, foot, back, and joint pains, continuous often intense neck pain that has been there since my twenties, along with severe insomnia and allergies, I arrived at 50 years old and began a quick descent into an abyss of deep and unexpected depression and anhedonia. I have been stuck here and have wanted to die 24/7 for 10 years, but haven’t because I do not want to hurt my grown son, and I am sharing the out of state caretaking of my mother and stepfather with dementia with my brother. I have been desperately trying for the last decade to recover my health. To that end, I have taken many supplements but none have really noticeably worked.
Attempts to Recover
Seven years ago, I took to injecting B12 after self-diagnosed pernicious anemia, but never felt a noticeable difference. I was extremely fatigued. I also injected a B complex regularly for several weeks or more without noticing a difference. I still feel a lot of fatigue but with the loss of motivation I think it is possibly more mental than physical.
Ten months ago, I began a strictly carnivore diet. Carnivore has helped inflammation. My bowels are way better and my lifelong mouth ulcers stopped immediately. There have been many other small wins. Unfortunately though, it means next to nothing to me because it has not fixed my depression, my enjoyment, or will to live. These are the core symptoms that I need to fix. I don’t understand why others get over their depression and insomnia and I cannot seem too. I also still loose lots of hair, but this has been going on for about 7 years. This is traumatic for me (constantly).
About three months ago, I experienced tachycardia plus dizzy spells for several days. The doctors said my iron was fine but I upped my heme iron and b12 and I think it helped. I eat a lot of liver/meat so it surprises me that I would ever be low in b12 or iron. I still feel a little floaty at times, but my heart rates are more normalized.
Recently, I discovered the literature and videos on high dose thiamine. I was very excited, and finally, once again hopeful.
I have taken both TTFD and benfotiamine for a couple weeks now and am not really noticing any changes paradoxically or feeling better. I recently added the HCL too. I have tried upping my doses significantly to where I was taking over 2000mg of Benfotiamine, 400mmg of TTFD and 400mg of thiamine HCL for several weeks. But I have since lowered it considerably. I also take magnesium (100mg), glutathione, riboflavin (100mg) the other B vitamins via yeast, B12 with intrinsic factor (500mg), and electrolytes, and I eat head to tail carnivore including bone broth. I take a substantial amount of more than 400mg of desiccated thyroid as well for the Hashimoto’s disease.
I started taking high dose niacin, perhaps a week ago and I think it kind of helped the thiamine. I felt a certain weight in my head lessen. It was not so much emotionally noticeable but like a bunch of swelling must have loosened. Then two nights ago, my body, legs, and some in arms, swelled up horribly. It was very itchy, painful and lumpy; like I had gained 20 pounds overnight. I haven’t had a history of noticeable edema. This scared me and I decided it was lymphedema, so I began doing lymph draining exercises. I finally felt it was not expanding any longer and perhaps even subsiding a day and half later. I felt hopeful that the brain inflammation FINALLY made a breakthrough, and my body was dealing with the toxicity that had been stuck in there, but I’m not sure what caused the sudden swelling. I also noticed during the swelling that I was urinating less, no matter my fluid intake. Perhaps my body was trying to dilute the toxicity and thus the necessary accumulation. I didn’t take the niacin or thiamine for the next two days. Then yesterday, I took a 1 gram niacin dose and felt a decline in the swelling, and later, around 3 am, I took another niacin, which somehow helped my body hurt less and I could relax. Now a few weeks later, the swelling has decreased considerably. I think it’s going to take some time to feel the results of brain regeneration and habitual behavior, but I don’t feel that feeling of a huge lump of coal stuck in my head anymore. I am currently taking 300mg of benfotiamine, 100 thiamine HCL and 100 allithiamine along with my minerals, electrolytes and vitamins. I also added oregano oil protocol that I heard could help with Hashimoto’s.
Please Help
I used to be highly creative and performed violin for a living, whereas now I cannot find any hope or inspiration to play or do anything and haven’t in years. I desperately want to clear the fog from my brain and regain my will. It is as if I am overwhelmed and underwhelmed at that same time. It is difficult to describe, except that I am miserable. What am I missing? Please any advice appreciated. Thank you!
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Photo by Annie Spratt on Unsplash.
This story was published originally on November 30, 2023.