We have many backup pathways in the human body. When one pathway is broken, another pathway can pick up the slack. If that second pathway is broken, that is when disease occurs. Oxalate is a potential second pathway breaker in individuals with underlying mitochondrial disorders. As a dietitian, I have found many of my clients, especially those with Autism Spectrum Disorder (ASD) or other neurodevelopmental syndromes, are struggling with oxalate, which then impairs their ability to metabolize vitamin A. My clients have symptoms of vitamin A deficiency and have retinoic acid deficiency, but because of poor NADH/NAD recycling actually have retinol and retinaldehyde toxicity; a conundrum to be sure, until one understands the connections between oxalate, NAD, and vitamin A metabolism.
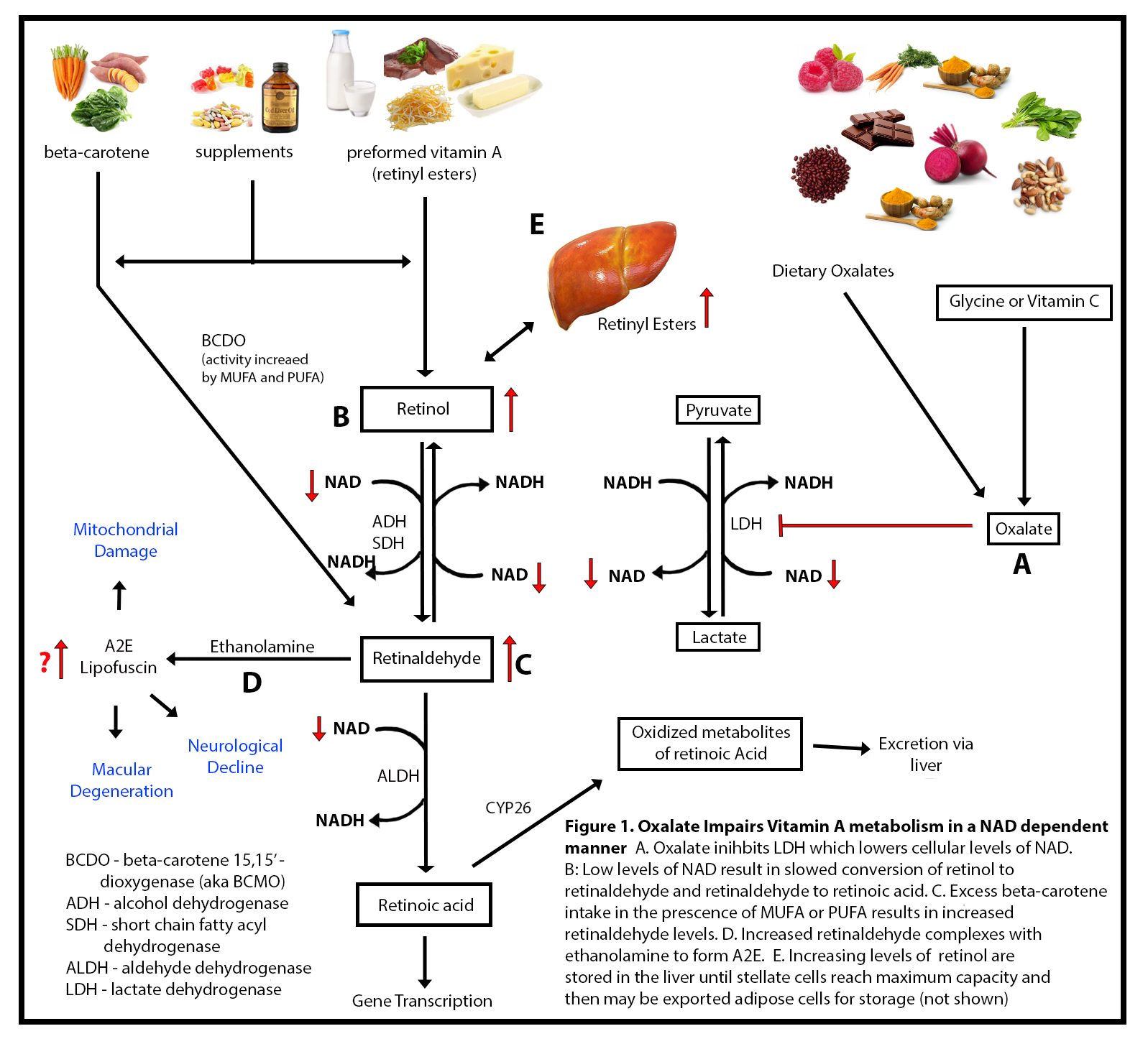
Briefly, nicotinamide adenine dinucleotide (NAD), derived from dietary niacin or vitamin B3, is a necessary cofactor in multiple enzymatic reactions involved in mitochondrial energy production. It is recycled endlessly back and forth between its oxidized and reduced forms NAD and NADH, respectively. The oxidized form NAD+ is required for the conversion of retinol to retinaldehyde and then to retinoic acid, the bioactive form of Vitamin A. With poor NADH/NAD recycling retinol and retinaldehyde are not converted to retinoic acid, and thus build up in the cell, presenting signs of both deficiency and toxicity simultaneously. The culprit behind this perplexing reaction, I believe, is oxalate damage to the NADH/NAD pathways via its interaction with an enzyme called lactate dehydrogenase (LDH). The chemistry in this pathway is a bit complicated, so bear with me. To help with the chemistry, I created a graphic (Figure 1.) to illustrate the pathways in question.
What is Oxalate?
The root of the dysfunction described above, I believe, begins with increased dietary oxalate and poor oxalate elimination. Oxalate is a component of plants that is impossible for the body to completely break down. It is a poison in large amounts. We absorb it at variable rates, but some of us make it in our bodies from vitamin C and glycine. Excess vitamin C becomes oxalate through direct degradation and without enzymes. Usually this occurs in vitamin C intake over 2000mg, but it can happen at lower doses as well. I never recommend vitamin C to “bowel tolerance” as this likely represents the death of the intestinal cells due to oxalate poisoning. Glycine is metabolized to oxalate in a B6 and thiamine deficient state, but when there is adequate B6 and thiamine, glycine does not become oxalate.
Oxalate Impairs Lactate Dehydrogenase
When oxalate is high, it impairs an enzyme LDH (Figure 1).We have to make some lactate to keep energy metabolism going, but when the mitochondrial respiratory chain is damaged, I believe this reaction becomes a more pivotal point for NADH to NAD recycling. While researching this possibility, Jenny Jones, a PhD in Human Molecular Genetics, shared this article to confirm my suspicions that the LDH reaction is a pivotal point of NADH to NAD recycling. The balance of NAD to NADH in cells is of the utmost importance to maintain normal cellular health.
When the body produces lactate, it also produces NAD+. This is what drives vitamin A (retinol and retinal) metabolism forward. What I found through researching literature, and through many of my clients experiencing hypervitaminosis A, is that oxalate does not directly inhibit alcohol dehydrogenase or retinol dehydrogenase or aldehyde dehydrogenase, which was what I thought originally, but rather, oxalate impairs lactate dehydrogenase (LDH), which then lower NAD+ levels. I hypothesized that oxalate takes away the “energy” needed to drive those reactions forward by impairing LDH.
Why Does Oxalate Inhibit LDH?
LDH is actually the last enzyme involved in the formation of oxalates. The benefit of oxalate being able to have a feedback inhibition on LDH is a safety mechanism to prevent oxalate poisoning. However, when dietary oxalate is too high this backfires and wreaks havoc on vitamin A metabolism and also energy metabolism. This means that high oxalate impairs LDH activity via NAD dependent pathway resulting in hypervitaminosis A in the form of retinol and retinaldehyde with a possible retinoic acid deficiency.
Oxalate Is Pathogenic in ASD
I mentioned in the introduction that oxalate has been implicated as a pathogenic substance in ASD. I propose that this is related both to a reduction in the NAD/NADH ratio which impairs overall energy metabolism, but also, due to the accumulation of retinaldehyde. High levels of retinaldehyde can form a complex with ethanolamine to form something called A2E in the skin and the eye. A2E is a lipofuscin (a pigmented by-product of failed intracellular catabolism) that has been found in the liver, kidney, heart muscle, retina, adrenals, nerve cells, and ganglion cells. It is studied predominantly in the eye as a symptom of advanced aging. In response to blue light, A2E increases reactive oxidant intermediates, but even in the absence of light, A2E has been shown to disrupt membrane integrity by acting as a detergent as well as by inhibiting key cellular function. A2E is just one possible way that poor vitamin A metabolism can contribute to altered mitochondrial function.
Retinoids and the various versions of vitamin A are mitochondrial toxicants when in excess. They cause the following problems due to alterations in cardiolipin (a stabilizer of the mitochondrial membrane) and displacement of cytochrome C oxidase. Cytochrome C oxidase, also known as complex IV, is the last enzyme of the mitochondrial electron transport chain before ATP is released. Damage to cytochrome C oxidase/complex IV causes all sorts of problems. The net effect of this mitochondrial membrane attack is:
- Increased free radical O2 production
- Increased nitric oxide (NO) which can then further impair cytochrome C oxidase
- Decreased ATP synthesis
- Decreased NADH to NAD recycling
I propose that the inhibition of these key cellular functions by aberrant vitamin A metabolism is causing the underlying inability to tolerate dietary oxalate in the absence of kidney stone formation, which then feeds back and causes more problems in the handling of vitamin A and dietary oxalate.
Since all of this is very technical, let me give you some real life examples of these patterns observed from family and my client base.
Case Evidence
My first clue to this pattern came from clients who rely on tube feeding where the food mixture contained high polyunsaturated fats and high oxalate foods such as carrots, sweet potatoes or almond meal of some sort. When the mixture contains high concentrations of beta-carotene (carrots, sweet potatoes) and is combined with polyunsaturated fatty acids, there is an upregulation of the beta-carotene monooxygenase (BCOM) enzyme leading to an increase in conversion of beta-carotene to retinaldehyde, as well as increases in cellular retinol binding protein 2 (RBP2) that facilitates uptake of vitamin A from the intestine.
Additionally, these foods are often also high in oxalate. The combination of a high oxalate tube feeding with an upregulation of BCOM and RBP2 leads to hypervitaminosis A of retinol and retinaldehyde with a deficiency of retinoic acid.
This pattern was not limited to just my tube fed clients. My daughter and four other clients also appear to have developed this pattern of high oxalate and hypervitaminosis A from their diets. In fact, regretfully, recommendations I gave to one of my clients, before I understood this pattern, pushed him into hypervitaminosis A with resulting liver failure and cognitive decline.
Client 1
For this client, I had prescribed a balance plate method for meal planning to help him with his cholesterol, triglycerides, and insulin resistance. I encouraged his family to cover half of his plate with vegetables at lunch and dinner to fill him up and provide more fiber. He loves carrots, so his lunch always had a large portion of carrots. Carrots are high in beta-carotene, but also oxalates. As I previously described, beta-carotene is a precursor to vitamin A but must be metabolized prior to being made into retinoic acid, the active form of vitamin A. It enters metabolism at the level of retinaldehyde, and in the presence of monounsaturated or polyunsaturated fat, is more readily converted to retinaldehyde. When combined with the oxalate induced downregulation of NAD/NADH pathway, this was a recipe for disaster.
With this diet, the client, who was already neurologically impaired, progressed quickly into dementia and now can no longer perform activities of daily living such as using a washing machine and changing his sheets. In addition, he now has elevated liver enzymes, metabolic acidosis, and insulin resistance. I had him checked for hypervitaminosis A by the only measure available, serum retinol levels. Indeed, his vitamin A levels were elevated. His intake of carrots in a NAD compromised state pushed him into worsening cognitive decline and liver failure caused by accumulation of vitamin A. Sadly, the balanced plate method is a standard diet prescribed to individuals with high cholesterol, triglycerides, and insulin resistance. It can be exceptionally high in oxalate and vitamin A creating a negative cycle of reactions that are damaging to health.
When we unpack his reactions, we can see that his liver toxicity can be explained by the fact that poorly metabolized vitamin A accumulated and contributed mitochondrial damage. This led to increased ROS which inhibited alpha ketoglutarate dehydrogenase. He was forced into a GABA shunt in the liver and also the brain leading to elevation in his AST and ALT enzymes as well as dysregulated behavior as his glutamate levels increased. However, because of an additional, what I believe to be, a functional B6 deficiency, he was unable to convert glutamate to GABA resulting in mood dysregulation from high glutamate and low GABA in the brain, as well as liver toxicity. GABA is an antioxidant for the liver and an inhibitory neurotransmitter in the brain.
As to his declining cognitive dysfunction, his physicians have told his parents that he has early Alzheimer’s disease. The current plan for this particular client to slow the degenerative process, improve cognition, and reverse liver disease by:
- Increasing dietary choline to restore cell membranes damaged by hypervitaminosis A
- Improve vitamin B6 status
- Reduce dietary carotenoids and insure Vitamin A intake is no higher than the RDA including any carotenoids.
- Avoid dietary oxalates in excess of 50 mg per day. This will need to be lowered slowly over several weeks to prevent a retinoic acid surge and symptoms of retinoic acid toxicity such as rash, hair loss, fatigue, nausea, and headaches.
Other measures, specific to his case will also be employed.
Aldehyde Intolerance Due to Oxalate: My Daughter’s Experience
Over the past 11 years, my daughter Zoey has had moments throughout the day, and sometimes the entire day, in which she walked as if she were drunk. She also suffered from what doctors wanted to diagnose as “cyclic vomiting syndrome”. Looking back, the more oxalate that she was consuming, the more ataxia I would see, and the worse gastrointestinal symptoms she would have (reflux, constipation, nausea, vomiting). She would also have extreme mood swings and behaviors over the past two years that were so extreme that her neurologist recommended she be put on a “mood stabilizer”. These were only symptoms of underlying impaired ability to metabolize aldehydes and the A2E ethanolamine steal altering her autonomic nervous system function. These are also the same exact symptoms that my clients, adults with intellectual disability of various origins, are having.
I have since learned the mechanisms of this apparent drunkenness: impaired aldehyde metabolism. This could be genetic due to polymorphisms in genes related to alcohol and aldehyde metabolism or due to a decrease in the NAD:NADH ratio in cells. Alcohol dehydrogenase and aldehyde dehydrogenase both require NAD to work properly. If someone consumes a high oxalate diet, they are likely impairing LDH and causing low NAD levels. This slows aldehyde metabolism and results in aldehyde toxicity. Aldehyde toxicity can cause local cellular deficiencies of sulfur-containing antioxidants including glutathione as well as local deficiencies of thiamine, pyridoxine, folate, zinc, and magnesium which further impairs metabolism causing oxidative stress and membrane lipid oxidation. Aldehydes are also capable of forming adducts with DNA and causing DNA damage.
This means that oxalate was indirectly impairing her ability to metabolize alcohol and aldehydes. We actually do make these consistently during metabolism, and so any disruption in NAD will impair clearance of these byproducts of metabolism. My poor girl has been drunk on her own metabolites! I wasn’t giving her sips of beer! And now that I am no longer accidentally poisoning her with oxalate, she is not running into walls as much or falling as much. It must feel good to not be drunk. She also has no more nausea or vomiting, and no significant reflux.
Increasing Retinaldehyde Levels Have Far Reaching Implications?
I mentioned previously that individuals with ASD may have altered vitamin A metabolism to the point of having high retinaldehyde due to high oxalate intake. I am currently in search of a research laboratory willing to explore this mechanism and whether it is a widespread issue or something specific related to genetic alterations. It may be that individuals with underlying neurodevelopmental disorders are just more susceptible to alterations in vitamin A metabolism which leads them down a worsening pathway of neurological decline. I believe people with genetic syndromes should be closely monitored for impaired vitamin A metabolism.
However, the effect of higher levels of retinaldehyde due to poor metabolism may be widespread. Alzheimer’s disease is at least in part due to altered vitamin A metabolism due to dysregulation of a crucial enzyme in retinoic acid synthesis, ALDH1A1. In fact, altered retinoid signaling has been implicated in Alzheimer’s disease. Retinoic acid is very much needed for normal brain function. Anger and emotional dysregulation can be a serious issue in individuals with Alzheimer’s disease. The midbrain relies on ALDH1A1 to convert glutamate to GABA and if it is tied up in retinaldehyde metabolism it may lead to impulsive behaviors. In Parkinson’s disease, ALDH1A1 is crucial for dopaminergic neurons and dysfunction of this enzyme can lead to loss of fine motor control and impaired working memory. It would be interesting to evaluate if high retinaldehyde can contribute to the alterations seen in Parkinson’s disease. In addition, there is indirect evidence that psychiatric disorders such as schizophrenia, bipolar disorder, and major depressive disorder are symptoms of impaired Vitamin A metabolism. Overall, more research is needed to evaluate whether impaired vitamin A metabolism and elevation of retinaldehyde levels is contributing to neurological related disease.
Dietary Intervention for Poor Vitamin A Metabolism
At this time, I am working with my clients on various aspects of improving their production and recycling of NAD to help improve their vitamin A metabolism. Often, this means encouraging them to speak with their doctors about changes in medications. Some medications can alter the ability to metabolize vitamin A. These include PEG laxatives with molecular weights less than 4000 (3.7% metabolized to oxalate in the body), H2 receptor antagonists (inhibit vitamin A metabolism in a NAD dependent manner), Metformin (impairs respiratory complex one recycling of NAD), and high dose melatonin (which leads cells into a high NADH state).
And, of course, dietary oxalate reduction plays a major role in our therapeutic efforts to get vitamin A metabolism back on board. For these individuals with hypervitaminosis A induced mitochondrial impairment, reduction of dietary oxalate has become a key tool in solving their inability to metabolize vitamin A due to low levels of cellular NAD.
We have many backup pathways in the human body. When one pathway is broken, another pathway can pick up the slack. I have hope that once the mitochondrial damage is repaired, my clients will again be able to have their carrot cake and eat it too!
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by Yerson Retamal from Pixabay.
This article was published originally on August 28, 2023.
















I’ve communicated with 2 real experts on Vitamin C and oxalate. One has written books about C and he insists that Vitamin C does not, NOT, make oxalate. Can we put up a good fight when we say Vit C does indeed transform to oxalate?
Contact Dr Thomas Levy for further information/refutation of this notion of Vit C transforming to oxalate.
Tremendous insight. There are two separate groups out there experiencing great health benefits from following either a low oxalate diet (TLO Facebook group) or a low vitamin A diet (Grant Genereux’ and Garrett Smith’s groups). Your article draws a connection between the two and also resonates with my own experience. Added on top of the points you make, is the fact that I have Gilbert’s syndrome, which involves limited capability of the UGT enzymes, which also happen to be suppressed by, you guessed it, retinol.
This is very interesting as I notice my palms are more orange when my fat intake is higher.
But I also noticed they are more orange when my dietary glutamate Intake is high.
I’d love to have a discussion on how this all relates to the Cell Danger Response, research from Dr. Robert Naviaux. I understand it’s about mitochondrial ATP and how calcium and glutamate act as danger signalling molecules, and the walls of the mitochondria harden until that danger has passed. This mitochondrial hardening can be the cause of so much chronic disease. Vitamins and minerals are inefficiently utilized in this situation, and I believe results in this deficiency-yet-toxicity situation noted so often. (like Vitamin A, B6, Copper etc.). I’ve had so many practitioners tell me I’m excess, but functionally deficient.
I’ve had childhood trauma that has continued to affect me into adulthood and had oxalate issues develop in my 40s. (when I was under massive work stress). I then retired and my work stress vanished, and I had no joint pain for two years until COVID came on the scene – resulting in a return of stress, and a return of symptoms.
I am proposing that both calcium and glutamate respond to stress ( which would deplete B6 ) and both contribute to oxalate issues. I’m hearing of so many people calming their nervous system through nervous system regulation exercises, and symptoms are vanishing… Is this due to glutamate and calcium returning to their designed function in helping to produce ATP? Look at the drug Memantine – block the effects of glutamate – and while approved for Alzheimer’s, it’s also showing to be affective with ADHD, anxiety, depression, long Covid, food bingeing eating disorders, all associated with glutamate in excess. And technically shouldn’t calcium be in the bones and not intracellularly where it’s available to bind with the oxalate? CDR – excess intracellular calcium.
Calm the glutamate, calm the calcium, mitochondria would soften, and minerals and vitamins would be absorbed. To me, our body’s innate intelligence all hinges on nervous system regulation for it to feel safe enough to get to work and do what it’s designed to do.
Would love your feedback. Because I am in CDR, Choline is a no-go at the moment…too much too soon?
Stephanie,
Thanks for commenting! I’m finding more and more that my adults with autism are intuitive eaters. The trick of blending veggies into their foods seems to be a huge “no no” at this point. So many of them have behaviors after meals. I was recently told by one foster mom that after having a meal of baby carrots, beans, and chicken spaghetti, my client went down hill at dayhab and acted out. Hmm…quite a large oxalate load.
This is so interesting that your son had cyclic vomiting and drunk behavior! I feel like that is very much oxalate related. Those almond based products! Zoey was drinking almond milk when I first realized her oxalate issue when she was 2 years old. I bet your son was protecting himself intuitively from extra oxalates and also from spikes in vitamin A.
Zoey will now tell me “blah..blah..blah” when she thinks a food will cause a reaction. She will still eat the food because she is metabolically starving. She seems to be only making energy from glycolysis. If she eats something high in oxalate (very rare now), she metabolically goes down hill quickly. She is actually scared of macaroni and cheese (butter and cheese are high vitamin A), but I make a low vitamin A version for her now. She will not touch chocolate anything.
Hypervitaminosis A and intolerance to dietary oxalate is starting to seem more like a symptom of underlying mitochondrial impairment, but then as vitamin A accumulates due to increased oxalate intake, Vitamin A becomes a source of mitochondrial impairment.
Very interesting. My son has categorically rejected fruits and vegetables since the age of 3. He is now 15. He has PANS and was once diagnosed with ASD, although I don’t think he would fit the criteria any longer. He did test high in oxlates when he was younger. Which is strange bc he didn’t eat much of them. His refusal of fruits and vegetables seemed sensory. He couldn’t be near anyone eating fruit because of the smell. But makes me wonder if the aversion is somehow the body’s way of telling you this isn’t good for you. He was gluten free so there was a fair amount of almonds in his diet from gf products.
He also, when younger, had issues with drunk behavior and cyclical vomiting.
I have another younger child who also has food sensory issues. She does not eat any vegetables and only a couple fruits. I havent had her tested for oxlates.