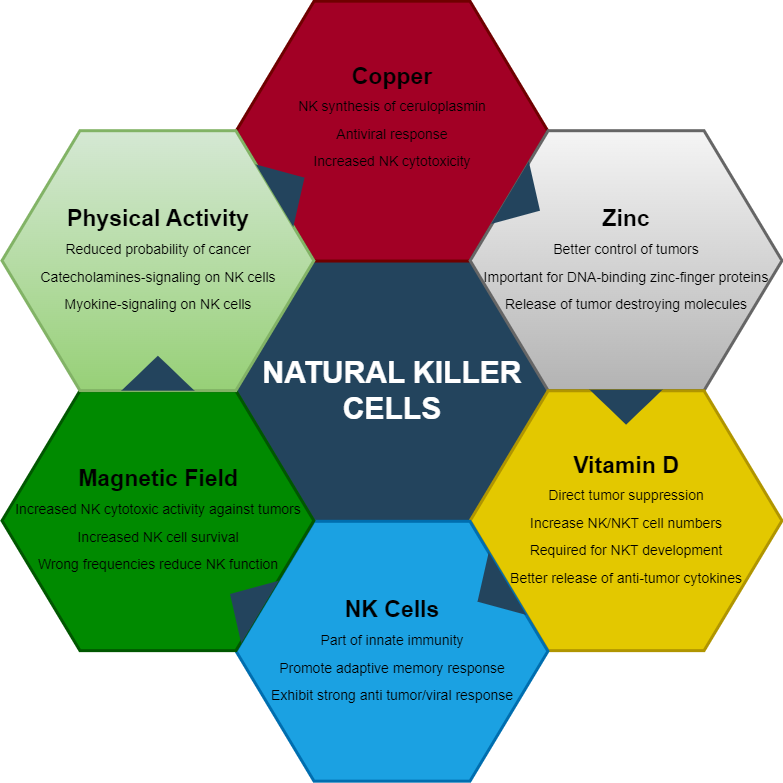
Natural Killer (NK) cells are an essential component of the human immune system and serve as part of innate immunity. These cells are built to seek and destroy virally infected cells, or cells displaying peculiar cancerous activity. NK cells’ development and function depend on complex networks of nutritional, biochemical, and environmental signals. Optimization of these signals through careful biomodulation will result in optimal NK cell development and functional activity against pathogens or tumors. The term biomodulation here will be defined as a change of cells or tissue in response to a therapeutic or pathologic stimulus. These modulations include nutrition, and systemic bodily perturbations.
A Short Primer on the Immune System
The human immune system is composed of a convoluted array of dynamic and static defenses. These include the skin, biochemical agents (e.g., antimicrobial defensins), and specialized cells that form innate and adaptive immunity. This intricate web of defense produces an environment built to protect the host from continuous onslaught by external, or opportunistic pathogens. Cells of the immune system are also involved in surveying bodily tissues for damaging insults acquired from the environment. Harmful insults could be chemical or radiation-induced bio-alterations that could lead to cancerous cell formation.
A dynamic faction of the immune system is the White Blood Cells (WBC) component – categorized under innate or adaptive status. WBC circulate through the blood network where they enter/exit tissues for surveillance. WBC form within the bone marrow from hematopoietic stem cells. For example, lymphoid precursors form adaptive lymphocytes, T and B cells. On the other hand, neutrophils, mast cells, and monocytes are formed from myeloid progenitor cells. Some WBC undergo further development stages, such as in the Thymus for T cells, before they are released. The latter allows for detection of cells that are either self-reactive (autoimmune), or non-reactive (do not function as well). T cells that do not pass these tests (receptor signals) are commanded to undergo apoptosis, or programmed cell death. All immune cells depend on a host of nutritional factors for development, activation, and effector functions. Malfunction or dysregulation of the immune network will result in autoimmune disorders, external and opportunistic infections, and formation of multiple types of cancers.
Innate and Adaptive Immunity – The Two Arms of Immunity
Innate immune cells are the first line of defense against infections. Innate cells harbor specific receptors which allow recognition of conserved pathogen patterns, or pathogen-derived chemicals. These receptors are termed germline-encoded pattern recognition receptors (PRRs). An example of PRRs would be Toll-like receptors (TLR) that recognize bacterial and viral components such as flagellin, lipoproteins, lipopolysaccharides (LPS), and ribonucleic acid (RNA). The responses of innate immune cells are relatively rapid and will occur within four hours of activation. However, although innate immune cells are essential, some pathogens can overcome their frontline defenses. Thankfully, adaptive immune cells eventually become involved so that they can “adapt” to these infections wherein long-term solutions are formed, in other words, formation of immune memory. However, before adaptive cells can form memory, they first need to be primed through an initial infection. In this case, adaptive cells (T and B cells) work in concert with innate cells to be primed and to form long-term memory against re-invading pathogens. This includes expansion of pathogen-specific T cells, termed clonal expansion. Importantly, B cells become antibody producing cells. The result is a reservoir of long-term circulating memory cells and antibodies poised for a fast response to infections – generally without the need of the initial discomfort when encountering new pathogens (fever, disease, etc.).
Natural Killer and Natural Killer T Cells
Natural Killer (NK) and Natural Killer T (NKT) cells are categorized under innate immune cells. NKT are special because they are viewed as a mixture of NK and conventional adaptive T cells (hence NK-T cells). NKT cells, however, are different from adaptive T cells because their T cell receptor can be activated by glycolipids. This is in contrast to conventional adaptive T cells that are activated by peptide (protein) complexes. NK/NKT cells play an important role in the defense against virus-infected and tumor cells. They patrol the body while integrating a diverse array of signals from cells and their environment. Once stressed cells are found (tumors or infections), NK/NKT cells become activated. Here, these innate cells destroy tumor or infected cells through direct receptor engagement, utilizing cytolytic granules and death receptors – the latter causing apoptosis.
Another important role for NK and NKT cells is to strengthen adaptive responses in-part by secreting immune-modulating compounds. The latter include cytokines [tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), granulocyte macrophage colony-stimulating factor (GM-CSF)], and chemokines (CCL1, CCL2, CCL3, CCL4, CCL5, and CXCL8). Nutrition is an essential component for the optimal function of NK and NKT cells. As we will see below, exposure of NK/NKT to nutritional compounds allows for better movement, optimized secretion of immunomodulatory chemicals, and enhancement of killing activity. For example, the importance of magnesium for NK cell function has been discussed previously on Hormones Matter. Herein, we focus mostly on NK cells with some insights for NKT cells.
Vitamin D Antiviral and Anti-Tumor Actions
The secosteroid, vitamin D3, is synthesized in the skin from 7-dehydrocholesterol upon exposure to electromagnetic waves in the ultraviolet-B region. Vitamin D2 is produced by irradiation of fungi and yeast, and both D3/D2 forms can be obtained from diet. Several modifications by cytochrome p450 enzymes in other tissues including, the liver, kidney, and lung epithelial cells produce the active forms, 1,25-dihydroxyvitamin D3/D2 ( 1,25D), which circulates as a hormone in the blood. The effects of 1,25D are mediated through binding of nuclear Vitamin D Receptor (VDR). Numerous cell types and tissues express VDR including the lung, kidney, bone, and lymphocytes. Upon activation by 1,25D, VDR influences calcium/phosphate homeostasis and metabolism.
The 1,25D/VDR axis results in cell signaling conductive to anti-viral and anti-tumor responses. Binding of Vitamin D to its receptors generates anti-microbial peptides by neutrophils and macrophages, and to enhance T cell activation via the T cell receptor. VDR signaling blocks the proliferation of human cancer cells, reduces lung metastasis in animal models, and is a preventive factor in the metastasis of lung cancer in humans. Interestingly, observational studies demonstrated an inverse correlation between circulating 1,25D or ultraviolet-B exposure and the incidence of lung cancer. In addition, increased dietary 1,25D improves results for lung cancer patients after surgery. Therefore, Vitamin D is a crucial component mediating anti-tumor responses through direct suppression of tumor cells, but also through the modulation of immune cells.
How does Vitamin D signaling affect NK cells? Mariani et al. showed a functional relationship between the numbers of cytolytic/cytotoxic NK cells and levels of Vitamin D. Although this is a correlation, we know that NK cells express abundant levels of VDR and can respond to 1,25D stimulation by upregulating and downregulating multiple genes. Therefore, we can see that vitamin D-induced stimulation directly affects NK cells. Other studies demonstrated that VDR signaling augments tumor susceptibility to NK cell mediated lysis. Interestingly, the cancerous growth studied were human melanoma cells. Here, 1,25D modulation increased specific proteins (FAS) on the cell surface of melanoma cells which then allowed for a better NK cell detection followed by destruction of the cancerous cells. Next, a clinical trial in ICU patients showed significant increase in NK and NKT cell counts after weekly treatment of 60,000 IU 1,25D. Another study showed that treatment with the active form of Vitamin D caused an increase in NK cell cytotoxicity after 1 month of oral treatment. The authors suggest the use of this secosteroid for immunomodulation in patients with low NK cell activity. We can extrapolate from these and multitude of other studies on Vitamin D, that increasing 1,25D levels during an infection produces a positive immunomodulatory effect for NK cells.
What About NKT Cells?
NKT cells are a major player in the anti-tumor response. For example, numerical defects in NKT cells are considered a poor prognostic marker in patients with colorectal cancer and neuroblastoma. In other words, lower numbers of NKT cells lead to worse cancer outcomes. Because they are so effective against cancer, NKT cells were used in immunotherapy, and these treatments were shown to enhance innate and adaptive immune response to tumors, and to increase serum levels of the immune-boosting cytokine IFN-γ in patients with various metastatic malignancies. IFN-γ is one of the cytokines released by both NK and NKT cells.
Do NKT cells receive the same beneficial effects from Vitamin D? Indeed, the effects of Vitamin D seem to even be stronger for NKT cells. The effects are seen from the developmental period whereby VDR knockout mice have fewer peripheral NKT cells due in part to a requirement for VDR signaling during development. In addition, VDR signaling regulates the expression of chemokine receptors which control NKT cell recruitment to different tissues. All of this highlights the importance of “indirect” VDR signaling for NKT cell function. Direct 1,25D stimulation can augment NKT cells’ ability to secrete cytokines through in-vivo VDR stimulation. Specifically, mice were fed 1,25D for 1 week and then injected with NKT cell stimulating compounds. The authors found that NKT cells from Vitamin D fed mice had significantly more IFN-γ and IL-4 production. Similarity, mice who had their VDR knocked out, had significantly less amounts of these cytokines. We can conclude here that vitamin D is important for the development and function of NKT cells, and that lower vitamin D levels will affect individuals in terms of reduced ability for development of strong and functional NKT cells.
Zinc Enhances NK Activity
Immune cells can regulate the influx and outflux of minerals depending on signals received from the environment. For example, in adaptive T cells, once their T cell receptor is activated, adaptive T lymphocytes begin by mobilizing sizable cytoplasmic and nuclear influx of multiple ions such as calcium, magnesium, and zinc. This activity leads to expression of numerous genomic pathways, including the secretion of IL-12 cytokine by WBC. Interestingly, one of the roles of IL-12 is to enhance NK cell activity. Therefore, zinc indirectly affects NK cells.
A 2018 study demonstrated that zinc supplementation enhances NK cell activity through increasing the expression of their cell-destroying proteins. Zinc also increased the effects of IL-2 on these cells, thereby acting as a synergistic molecule to this important survival cytokine. On the other hand, zinc deficiency reduced NK cell activity and dampened the effects of IL-2. Another study showed that mice deficient in zinc had poor control of liver tumors. In contrast, too much zinc showed an inhibitory effect on NK cell cytotoxicity – demonstrating a potential zinc-induced imbalance (possibly through reduction of copper).
How does zinc regulate immune cells? By scoping the genomic level, we can find that zinc is an integral component of how the cell regulates transcription from DNA. Transcription is the process of making messenger RNA (mRNA), and other types of RNA, so that the cells can respond optimally to danger signals. Here, zinc is part of specialized protein structures termed Zinc-Finger protein domains. These domains require zinc ions in order to stabilize the amino acid structure so that the proteins maintain an appropriate 3-dimensional form. These Zinc-Fingers are part of larger proteins that bind with DNA, some of which can be named transcription factors. Together, these zinc-based structures play an essential role in DNA regulation. For example PLZF, a zinc-finger transcription factor, was shown to be involved in NK and NKT cell development. This is so important that immune cells make sure to express specific zinc transporters to capture this ion inside the cell, so that enough ions are available for protein function when danger signals arrive.
Copper, Ceruloplasmin and Hematological Dysfunction
We have previously discussed the importance of copper with regards to thyroid and mitochondrial function – all of which are important for proper immune function. Copper deficiency is an established cause of hematological abnormalities and can present in an almost identical pattern to myelodysplastic syndromes. Myelodysplastic syndromes are a group of cancers involving immature blood cell formation. When copper levels become chronically depleted, these blood abnormalities can show up as mono- bi- or pancytopenias (lower than normal red or white blood cells), with the neutrophil (innate cells) lineage sometimes being the most susceptible to low copper reserves. But is there evidence for copper-mediated mechanisms directly on NK cells?
Let’s first take a look at a case study for copper deficiency. Bhat et al. recently presented a case of a 2-year old patient exhibiting symptoms of Menkes disease (low serum copper and ceruloplasmin, hypotonia, kinky hair, and developmental regression). Menkes disease was then confirmed by genetic assessment. The treating team commenced on a regimen of copper chloride, which produced positive results (more alertness, and better movements). This patient had suffered from recurrent respiratory infections, in-part due to a substantial dysfunction of NK cells. Given this observation, they postulated that copper is needed for NK cell activity (and probably development). The latter has been directly shown in a 1987 study whereby rats fed a copper-deficient diet had lower antibodies and NK cell cytotoxicity. Certainly, it would make sense to assume here that these defects are due to a reduction of energy and mitochondrial function, needed for proper NK cell function.
Clues about mechanisms behind copper-mediated regulation of NK cells come from another research group, Banha et al. They demonstrated that NK cells express the multi-copper protein, ceruloplasmin. Ceruloplasmin (also named ferroxidase I) carries around 95% of the copper found in plasma. It is required for the oxidation of ferrous iron to the ferric forms. This process allows the incorporation of ferric iron into the protein, transferrin, and eventually hemoglobin. However, relative to other immune-related proteins, not a lot is not about ceruloplasmin in general, other than its role in binding and transporting copper.
Banha et al. showed another potential role for this copper binding protein by performing mRNA and protein studies on human derived WBC. Their experiments revealed a higher density expression of ceruloplasmin specifically with NK cells, compared to other WBC. Even the closely similar NKT cells did not display as much ceruloplasmin expression. Interestingly, the experiments revealed that human WBC express the secreted and membrane bound versions of ceruloplasmin. These experiments highlight a secondary role for ceruloplasmin outside of the liver and plasma. Observation with NK-associated ceruloplasmin could elucidate why the Bhat et al study with the 2-year-old child showed a dysfunction of NK cells, as ceruloplasmin is a major copper binding/transport protein. Taking the two studies together we can postulate here that immune cells utilize ceruloplasmin to harbor and release copper around infection sites in order to boost immune activity in a local infection or tumor sites.
Movement and Exercise Improve Immune Function
There is little doubt that movement and exercise are one of the best health-improving tools. For example, in sprints, just the jarring of the skeleton produces a stimulus in bone forming cells. The shock-waves and force implemented when bone and ground meet alter the back-and-forth dynamics between the osteoblast (bone forming cells) and osteoclasts (bone absorbing cells) – inducing bone density increase. In addition, the body is frantically signaling to itself to increase mitochondrial power and bio-genesis.
The benefits of movement are not solely limited to bodily power and output. The immune system, and in particular NK cells, receive some of that energy from exercise. In fact, NK cells are one of the most responsive cells to exercise with regards to mobilization from body tissues into circulation. NK cell mobilization is associated with exercise-dependent protection against cancer. Given their strong anti-tumor activity, mobilization of these cells likely allows for better trafficking with optimized seek & destroy activity. Of note, exercise seems to increase only the mobilization and not necessarily the production of new NK cells.
Just how much exercise is needed to receive these benefits? Although different types of movements can produce this mobilization effect, movement can be as little as a small flight upstairs. Millard et al utilized 29 volunteers and drew their blood before and immediately after exercise to study the frequency of their WBC. They showed that as little as 150 stair steps will increase the numbers of NK cells in the blood by 6 fold. Interestingly, females had a larger increase in the numbers of NK cells than males. Another work by Timmons et al showed that maximal NK cell mobilization occurred at 30 minutes of endurance training, after which no increase was observed. This effect on NK cells by exercise has been attributed in-part to the release of catecholamines (epinephrine and norepinephrine). Direct evidence of catecholamines-induced NK mobilization was displayed, when systemic administration of catecholamines produced similar effects as exercise-induced increased of NK cell. What is also important is that mobilized NK cells had higher cytotoxicity – so they performed better against tumors. Why do NK cells react so well to catecholamines? It is because they express the highest amounts of beta-adrenergic receptors (receptors for catecholamines) of all WBC.
It does not all stop with catecholamines, however. Myokines, which are cytokines derived from muscles during exercise, become involved in regulation of mobilized NK cells. Myokines are known to stimulate immune cells and they include IL15, IL7, and IL6. Thus, NK cells are involved in an intriguing immune-to-muscle crosstalk during exercise. The mobilization and optimization of functional activity of NK cells by myokines during exercise is believed to explain why exercise can be so beneficial in a cancer setting.
Magnetic Forces to Harness NK Potential
Environmental impacts of NK cells are not limited to just different forms of exercise. Magnetic forces can be utilized to harness the power and potential of NK cells. Modulatory effects of static and dynamic magnetic forces on many cell types, have been well known for decades. With respect to NK cells, their activity is potentiated by static magnetic fields. A relatively recent work by Lin et al. showed that perturbation by a 0.4 Tesla static magnetic field increased the viability and activity of human NK cells. If you are wondering, 0.4 Tesla can be classified as moderate level static magnetic field which is below two other levels (strong & ultra-strong fields). Magnetically treated NK cells showed significantly more killing activity compared to non-magnetized cells, after incubation with K562 tumor cells. The effects of magnetism on NK cells have been attributed to increased receptor activation pathways (MAP Kinase). These results indicate to us that proper magnetism can be beneficial to a variety of diseases (including cancer).
Manipulations by static and dynamic magnetic fields on all cell types, though no doubt produces measurable effects, have nevertheless been conflicted with varying and sometimes opposing results. The issues stem mainly from researchers using different experimental procedures and protocols. The latter includes, field strength, frequency and duration of the magnetic fields, target cells, and distance from magnets. In addition, cells tend to respond differently depending on their stages of growth and types/strength of magnetic fields. For example, testing on guinea pigs showed suppression of killing activity of NK cells when treated with a 50 hertz electromagnetic field. Interestingly, the same strength electromagnetic field causes DNA perturbation resulting in activation of latent Epstein Barr Viruses, a prime target of NK cells! Therefore, some frequencies not only activate viruses, but also result in suppressing the same cells required for removal of these pathogens. On the contrary, another study revealed positive effects on rat NK cells when treated with electromagnetic waves. Specifically, NK cell cytotoxic activity increased by 130-150%. Other types of immune cells have also shown a similar behavior when activated under magnetic fields. For example, moderate level static magnetic field perturbation caused human CD8+ T cells (adaptive immune cells) to increase mitochondrial respiration, ATP production, and increased immunomodulatory secretions.
There is no doubt that electrical and magnetic forces affect how our cells behave. It seems that moderate level static magnetic fields tend to promote beneficial activity for innate and adaptive immune cells. However, much more research is needed for specifying the mechanisms of these forces and to decipher beneficial and harmful parameters. Important parameters to elucidate would be A) how do perturbations of static and dynamic fields differ in their effects, and B) what doses are appropriate. Overall, our cells clearly respond to electromagnetic signals, but more work is needed to show how we can use these fields to promote immunity.
Conclusion
The role of NK cells in protecting our cells from viruses and tumors is clearly established. To support these cells in these important functions, we must provide important nutritional and physical ingredients. A deficiency in zinc not only affects several bodily and structural functions, but also suppresses the immune system, including NK cells. Similarly, both copper and Vitamin D are needed for development and promotion of NK cell-mediated anti-tumor activity. Production and anchoring of ceruloplasmin on NK cells’ membrane, is an indication of the importance of copper for NK cell function. Other, not so clearly elucidated mechanisms, such as magnetic fields seem to be an important component in the convoluted web of signals that NK cells receive. The above discussion also highlights how too much or too little of anything can compromise this web of signals. Too much zinc will alter copper metabolism, and the wrong electromagnetic signals can promote viral expression and suppression of immune cells. The reader is therefore advised to embark on a journey to optimize the immune system through implementation of calculated nutritional and physical mechanisms.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, and like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Feature image purchased from iStock, #18165875.
This article was published originally on March 23, 2023.















