When people have had a prolonged deficiency in energy metabolism, sometimes for years, their symptoms are frequently not recognized for what they represent. Because energy is required for every cell in the body, the symptoms are caused by how many cells have become dysfunctional. The brain and heart are the organs that dominate the consumption of energy, explaining why changes in behavior, organic brain disease and heart disease are so common. The symptoms, referring to the complaints of the patient, are all interpretations by the brain. By far and away the commonest symptom is chronic intractable fatigue and the accompanying symptoms have given rise to a common diagnosis called Chronic Fatigue Syndrome (CFS). A patient has been described whose CFS was found to be due to a genetic defect in the mitochondria, the organelles within the cell that produce energy. How often there is a genetic risk is unknown but the new science of epigenetics tells us that genes seldom work on their own. Another factor (malnutrition, stress) usually comes into the equation in order for the gene to be expressed in disease.
Anabolic and Catabolic Metabolism
Metabolism is the name for the sum of chemical reactions taking place in the human body. Because some reactions break large molecules into smaller pieces, while other reactions build up larger molecules from constituents, metabolism subdivides into two categories, catabolic and anabolic. Anabolic metabolism refers to reactions that build up molecules while catabolic metabolism breaks them down. Anabolic processes require energy derived from oxidation of food, while catabolic processes release energy by the oxidation of the molecules derived from constituents as they are broken down. These two functions must necessarily be in relative balance in a fully grown healthy person because, when growth is completed, a steady body weight results. Anabolic function dominates in the growth of a child, becoming balanced when growth is completed. In a lifespan, normal energy metabolism derived from oxidation of naturally occurring food substances gradually deteriorates and during aging, catabolic metabolism begins to dominate the balance, explaining the tendency to shrinkage in the elderly person. Anything that affects the oxidation of food substances in energy production results in an abnormal balance between the two types of metabolism. Thus, starvation, an improper ratio of calories to non-caloric nutrients, or genetically determined factors result in dominance of catabolic metabolism.
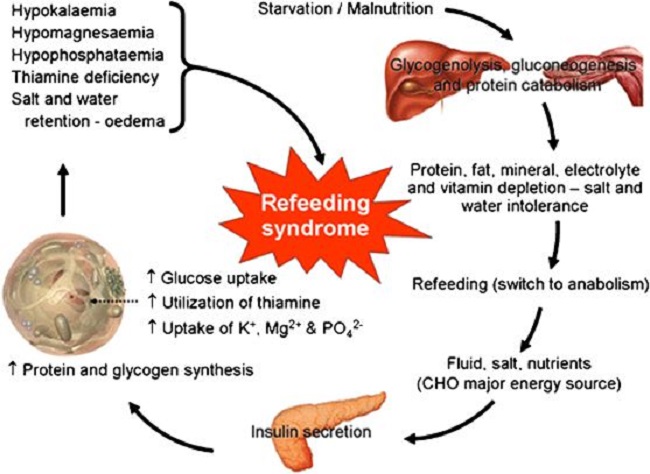
Refeeding Syndrome
Refeeding syndrome is what happens when an individual’s long-standing state of catabolic metabolism is too rapidly treated with the necessary nutritional ingredients to restore the metabolic balance. Attempts to treat the chronic starvation of incarcerated victims in the Nazi -controlled camps after World War II resulted in death in many of them. It is a dangerous condition that occurs with reestablishment of adequate nutrition in malnourished and cachectic patients. More specifically, its occurrence has been reported during oral enteral and parenteral refeeding. I remember the case of a boy who was a “junk food junky”. He had just come down from a rope that he had been climbing in the gymnasium, illustrating his apparent physical fitness, but he then suddenly passed out. He was taken to a local hospital by ambulance when he was given glucose saline intravenously, a standard procedure. He had a series of bloodstained bowel movements and expired. We have known for years that introducing glucose intravenously to a long-standing thiamine deficient individual is dangerous. But how would this come to light in an emergency situation? To prevent the possibility of refeeding syndrome would demand the reason for this young man passing out be known in relation to his junk food diet.
Clinical features of refeeding syndrome are fluid-balance abnormalities, abnormal glucose metabolism and a deficiency of magnesium and potassium. In addition, thiamine deficiency dominates. Refeeding syndrome reflects the too rapid change from catabolic to anabolic metabolism, is often undiagnosed and some clinicians remain oblivious to its occurrence. Recognition reduces morbidity and mortality but there is no universal agreement as to its definition. A report exploring refeeding syndrome across seven cases found that each showed deficiencies and low plasma levels of potassium, phosphate, magnesium and thiamine combined with salt and water retention. It is interesting that salt and water retention are typical of thiamine deficiency. Similarly, a report exploring the incidence of Wernicke’s Encephalophy with anorexia nervosa found that 8 of 12 cases of anorexia investigated were afflicted with the full symptoms of the thiamine deficiency brain disease known as Wernicke encephalopathy. Anorexia affects 2.9 million people worldwide and is generally considered to be psychiatric loss of appetite. Both its active state and refeeding can be lethal.
Guidelines for refeeding patients with anorexia have been published by the European Society of Clinical Nutrition and Metabolism. Of 65 in-hospital patients studied, 14 were admitted more than once within the study period. Nine patients had minor complications in the first 10 days of replenishment. Four patients had transient pretibial edema (simple pressure with a finger below the knee resulted in dimpling). Three patients had what was described as organ dysfunction and two patients had severe hypokalemia (low potassium in the blood), all of which have been described as typical of thiamine deficiency. In fact, pretibial edema can be the only clinically obtainable evidence of thiamine deficiency. There is a high prevalence of thiamine deficiency in cancer patients. The prevalence of malnutrition is high in head-neck cancer patients, many of whom require artificial nutritional support or refeeding intervention. Refeeding syndrome is commonly encountered in the nutritional treatment of critical illness. However, guidelines and its occurrence in ICU patients remain unclear. Calorie restriction for several days and a gradual increase of its intake has been recommended. Thiamine deficiency brain disease is not too uncommon in parenteral nutrition. It has been reported that refeeding syndrome occurs in 4% of cases of parenteral nutrition, but failure of its recognition occurs in 50%.
Refeeding, Paradoxical Reactions, and Side Effects
Discerning readers of Hormones Matter have probably noticed that the subject of “paradox” has been mentioned a number of times and some time ago there was a comment that an article on the subject might be relevant. “Paradox” is a less severe form of RFS, a term that I have used to indicate that the patient’s expectation of improvement by nutritional replacement is often dashed because the symptoms become worse. Obviously, because pharmaceuticals are the usual and customary form of treatment, the worsening of symptoms immediately gives rise to the patient’s deduction that these are side effects. In a sense, they are indeed side effects, but the mechanism is very different from that caused by pharmaceuticals. The accentuation of symptoms represents a sudden switch from the prolonged state of catabolic/anabolic balance to that of anabolic/catabolic balance, whereas side effects of drugs are a direct effect of toxicity. This accentuation of symptoms seems to be directly related to the chronic nature of the malnutrition. It means that the unfortunate patient has been suffering for an extended period without the symptoms being recognized for what they represent. If the symptoms are correctly diagnosed at the outset of symptoms, the nutritional correction is easy and occurs rapidly. Paradox is because recognition comes after protracted malnutrition and is much more likely with intravenous nutritional correction. I always warned the patient before administration. However, the paradoxical worsening of symptoms may last as long as a month when vitamin therapy is used in oral administration. I have always told the patient that paradox is the best sign of ultimate improvement. For example, I was discussing the common symptoms of high calorie malnutrition with a nurse. She interrupted by telling me that I was describing the symptoms that she had been suffering for years. I suggested the nutrient replacement and she told me later that paradox had lasted for a good month but was then replaced with an absence of symptoms and an energy level that she had never previously experienced.
Modern Malnutrition
Readers must understand that chronic long-term malnutrition is common in America. However, it is not the same as the kind of malnutrition that is seen for example in Bangladesh, or that seen in advanced cancer, known as starvation. The kind of common malnutrition in America is because of an excess of calories and is often seen as an oxymoron. How can a high calorie diet possibly cause a potentially severe illness? The clinical expression of starvation is that of bodily attrition through catabolic breakdown leading to death, the kind of clinical situation underlined by Mother Teresa and caused by lack of any sort of food. People with high calorie malnutrition look entirely different and often constitute what I call the “walking sick”, because they are commonly seen as “problem patients” in the offices of physicians. They are often obese and their many complaints are most often diagnosed by physicians as “psychosomatic”. Their problem is too much food of the wrong sort. Thiamine/magnesium levels in the blood are usually perfectly normal if they are ever measured, giving rise to a physician’s refusal to diagnose the “absurd idea of a vitamin deficiency”. Thiamine activity is inside cells, so finding it in the liquid part (plasma) of the blood is meaningless. Like a “choked car engine” the non-caloric nutrients are overwhelmed by the excess of “empty” calories. It is energy production that is the core issue and is the reason for the multiplicity of symptoms. This is particularly true for deficiency of thiamine and magnesium because they are so essential to the processing of simple sugars. The indiscriminate ingestion of sweets has become a national calamity. Of course, thiamine and magnesium have to cooperate with many more non-caloric nutrients but their position in metabolism dominates the function of energy production.
So, high calorie malnutrition is an example of the effects of extremes, too much versus too little. The brain, heart and nervous system are the most affected organs because of their high energy requirement. The commonest symptom is fatigue but other common symptoms include “brain fog”, insomnia, a perpetual sense of anxiety or fear, heart palpitations, migraine, tension headaches, poor tolerance to heat and cold, unusual sweating particularly at night, diarrhea alternating with constipation, pins and needles in the extremities and vicarious body and limb pains. Because physicians in America have denied the possibility of vitamin deficiency disease, they usually interpret any abnormal laboratory studies to what they consider to be well-recognized and common diseases such as chronic fatigue syndrome. If lab studies are normal, then it is deemed to be “psychological”, a very unsatisfactory explanation to the patient.
When a person has been consuming simple carbohydrates in the form of sweets, including carbonated beverages and alcohol for a prolonged period, the general efficiency of cellular metabolism gradually declines. The three meals a day in some cases may be perfectly adequate but the dietary excesses may come for that person because of “the goodies” associated with social activities. Because the brain is most affected, the symptoms will be generated by the biochemical and electrical changes that follow. The symptoms are so variable that listing them all is virtually impossible. It has long been known that beriberi, the clinical expression of thiamine deficiency, had a long morbidity and a low mortality. The suffering experienced during its prolonged course was (and still is), however, an abysmal reflection of medical ignorance.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
This article was published originally on July 29, 2019.
Rest in peace Derrick Lonsdale, May 2024.