For the last forty years or more, the fate of cells was believed to be predominantly, if not solely, determined by genetic blueprints. Everything from the earliest stages of gestational development to the expression of diseases like cancer was, and largely still is, considered genetically determined. Environmental considerations, while acknowledged, are believed to play a much lesser role.
As the genome was mapped, the medical industry, especially those in the field of cancer research, held out great hope for identifying the genetic origins of disease, only to be let down repeatedly. Researchers failed to link up to 95% cancers to any genetic defect and the frequency of genetic defects associated with the vast majority of non-cancer related disease processes was found to be less than a measly one percent. To accommodate the discrepancy between expectation and data, random chance entered the conversation. Researchers argued that what could not be accounted for by genetics or the few environmental causes considered, must be the result of randomly generated mutations; stochastic events for which we have no explanation. A study published in 2015, supported that claim.
These results suggest that only a third of the variation in cancer risk among tissues is attributable to environmental factors or inherited predispositions. The majority is due to “bad luck,” that is, random mutations arising during DNA replication in normal, noncancerous stem cells.
It is important to understand what randomness means in this context. Here, when genetic variations arising from computer simulations cannot be attributed to either known genetic and/or recognized environmental associations with cancer, they are classified as random. In other words, what is unknown or unrecognized within the confines of the experiment is considered random. As one might imagine, this creates a few problems. Most notably, we don’t know what we don’t know. To say with any certainty that something is random suggests all variables are known and accounted for within the given model. This is just not so, especially with regard to the environmental contributions to illness. Here, not only are the recognized carcinogens limited, but the manner in which we consider these and other non-genetic variables relative to cancer or any disease process is flawed, and ultimately, biased. What is considered environmental in this context, for example, is poorly defined.
In cancer epidemiology, the term “environmental” is generally used to denote anything not hereditary, and the stochastic processes involved in the development and homeostasis of tissues are grouped with external environmental influences in an uninformative way.
That said, in the aforementioned study specifically, the apparent randomness was shown to be associated with the number of stem cell divisions in particular tissues. Tissues with highly replicative stems cells were more likely to generate ‘random’ genetic variants and thus cancer. Mathematically, and indeed, intuitively, this makes sense. More activity equals more chance for error. We see this all of the time in machine learning and computer simulation experiments where random errors occur based upon the number of calculations. We also see this in basic mechanics where increased activity means more wear and tear.
I would venture that in biological systems, however, where life interacts with itself and its ‘environment’ continuously, dynamically, and non-linearly, the randomness posited by the linear probabilities observed in computer simulations may not accurately represent real world response. Perhaps the association between increased stem cell activity and cancer is moderated by other variables that we have yet to fully appreciate. In this particular study and likely others as well, the increased randomness was identified in precisely the regions that interact more closely with the environment. In other words, to use computer parlance what we are calling randomness may be a design feature rather than a bug. Would it not make sense that these regions would require increased turnover compared to other tissues more removed from direct environmental interactions? Might these tissues also be exposed to significantly more toxic insults and thus demand more energetic support e.g. nutrients, to mitigate both the potentially damaging effects of these exposures and the increased turnover? I suspect that the unmet demands of metabolism are more directly responsible for these seemingly random events. That said, perhaps instead of relegating these non-heritable mutations to the trash heap of randomness, we ought to look more closely and how ‘environment’ interacts with genetics.
Enter the field of epigenetics.
Epigenetics and Disease
As genetics fell short, an adjacent field, called epigenetics, gained steam, in some circles at least. Epigenetics technically means ‘over’ genetics. In this field, researchers look at the chemical variables that influence genetic expression without altering DNA itself. Specifically, they look at the addition of histones or methyl groups to DNA molecules, chromatin remodeling (how genetic information is organized), and changes to non-coding RNA (RNA proteins that don’t alter genes but affect their expression) to DNA molecules. Someone called these proteins ‘DNA decorations’ – a good descriptor, I think.
While these ‘decorations’ do not affect the core structure/arrangements of DNA like traditional mutations do, they are not without consequence. They activate or deactivate gene expression disrupting normal regulation. This means that whatever function the code from that gene performs, the epigenetic marker will either turn it on or off, constitutively, and generally, outside of the bounds of its normally regulated activity. As one can imagine, this can be problematic, particularly when a necessary function is re-regulated in a manner that negatively impacts cell fate during development or across the lifespan, as with cancer.
Fortunately, these changes are not necessarily permanent. They are malleable and dynamic. To that end, once the stressor is removed, so too is the epigenetic marker, at least in theory and in research situations. There are indications of lasting epigenetic memories, however. Epigenetic memories act like a DNA conditioning factor of sorts to prolonged stress such that new stressors more readily activate these patterns. Importantly, these epigenetic patterns and memories are heritable, suggesting that the stressors of our parents and grandparents decorate our DNA and permanently alter how our bodies respond to stress. Consider the research on Irish and Dutch famines where developmental malnutrition is linked to epigenetic markers associated with certain disease states generationally.
Notably, unlike in the field of genetics, were the incidence of mutations accounts for only small percentage of disease, epigenetic alterations in gene activity may account for 90% of variability of human disease. In this regard, epigenetics explains, at least broadly, why, in people with the same genetic defects, only a small percentage go on to develop cancer or any other disease processes linked to that gene defect.
As one might expect, there is an enormous and varied compendium of possible triggering factors with everything from toxicant exposures to poor nutrition and aging included. It appears that any environmental stressor or repeated behavior initiates changes to the epigenome, including more positive variables like good nutrition and exercise. This makes epigenetics an important interface between genetics and the environment. It does not appear to be the only or primary interface, however. For that, we have to dig a little deeper and ask ourselves, what is capable of driving both genetic and epigenetic activity? You guessed it. That power and responsibility resides with mitochondria.
Mitochondria speak the language of the epigenome. All substrates and cofactors required for epigenetic modifications of the DNA and histones are made by or metabolized by mitochondria. – Martin Picard
Everything Comes Back to the Mitochondria and Nutrition
Research over the last few decades shows that both genetics and epigenetics are the handmaidens of mitochondrial metabolism and not the other way around. And this make sense, because mitochondria are responsible for using nutrients to synthesize ATP – energy – and other important molecules that form the backbone of survival. No energy, no life. Everything from the proper unwinding of genetic code through the aberrant growth of cancer cells is determined by metabolic capacity, or more bluntly, nutritional availability. Across species, the patterns are conserved. From the earliest stages of cell development and across the lifespan, cell fate is determined by mitochondrial metabolism.
Developmentally, a growing body of research, shows just how clearly metabolism affects cell fate. An experiment using the single celled organisms called dictyostelium, the lowly slime mold, illustrates what happens when nutrients are absent. Here, when the organism has sufficient nutrients, it grows and reproduces into other single celled organisms – as expected – but when nutrient starved, it releases mitochondrial damaging reactive oxygen species (ROS), and eventually, develops into a multi-celled clump that travels to find nutrients. Nutrient availability thus, changes the fate of the cell, profoundly. Essentially, the genetic blueprint of the organism tells it to look and behave a certain way, but when genetics interacts with the environment, environment makes the final decision. In this case, in order to survive the lack of nutrients and the abundance of ROS produced by the lack of nutrients, the organism divides into multiple cells. This patterned is reproduced, more or less, in all organisms, even mammals.
Under nutrient rich situations, ROS molecules are leaked by the mitochondria whenever ATP/ energy is made. ROS are signaling molecules. As a signaling molecule, it is both necessary and regulated. Both too much and too little are problematic and thus there are other molecules and feedback mechanisms to manage its synthesis. The anti-oxidant glutathione is one the molecules charged with managing ROS.
In nutrient rich situations, mitochondria will produce energy and a supply of other important molecules that all work together to maintain the life and functioning of the cell. Regular mitochondrial replication and controlled apoptosis cycles are also involved and ensure a ready supply of healthy mitochondria capable of producing these molecules.
In a nutrient starved environment, however, there are not enough resources to maintain replication cycles and defense mechanisms simultaneously, so the cell has to make some decisions. In the case of the slime mold, and in fact, in every eukaryotic cell in any given organism, resources are shunted to maintaining defenses, into producing anti-oxidants like glutathione. Energy that is normally produced in the mitochondria, is now produced mostly in the cell itself (glycolysis), which is far less efficient, and replication and apoptosis cycles are upended. In other words, in resource poor environments, defense systems are favored over everything else. In this case, and with cancer, unbridled cell division is the defense mechanism.
Cell Fate Decisions In More Complex Organisms
While fascinating, it is difficult to appreciate the importance metabolism in cell fate decisions when the evidence comes from slime mold, but the patterns are conserved in more complex systems as well. Consider the mouse for example, where the effects of nutrient starved mitochondria are no less compelling. When nutrient dependent components of the mitochondria are blocked during early development, the cells initiate a stress response and stem cell specialization fails. Skin, lungs, and other tissues are poorly formed and the animal dies.
Research involving the earliest stages of life, the pre-implantation period from 1-2 cells for mice (up to 4 days) and 4-8 cells in humans (~6 days) clearly demonstrates the role metabolism in determining cell fate. Embryonic stem cells require mitochondrial metabolites, which depend upon key nutrients, to develop. When absent, development is arrested, sometimes irrevocably.
Importantly, when sperm and oocyte mix and life begins, it is not genetics, per se, or even epigenetics that guide cell division, cell fate, and subsequent embryogenesis, but energetic capacity – metabolism. Backing up even further, mitochondrial capacity and the local environments of both sperm and oocyte determine whether and how the two will meet. In essence, mitochondria serve as a bridge between energy metabolism and the epigenome, providing signals that can modify DNA and histone modifications, ultimately affecting gene expression and cellular function.
And then there is thiamine
When we unpack the patterns a little bit more, we see that the metabolite pyruvate is critical to this process. Recall from our discussions on mitochondrial function and nutrition, that pyruvate drives mitochondrial energy production. It is the end product of glycolysis (cytosolic carbohydrate metabolism) that, when in the presence of sufficient oxygen and thiamine, enters the mitochondria and is converted into acetyl-CoA, which after more reactions eventually becomes ATP – energy. Pyruvate, it appears, is required to activate the zygote genome. How it does this, is fascinating.
During early development (from 2-8 cells), mitochondrial enzymes from the first half of tricarboxylic acid (TCA) cycle (pyruvate dehydrogenase – PDH, pyruvate carboxylase, citrate synthase, aconitase 2, isocitrate dehydrogenase 3A, and a-ketoglutarate dehydrogenase – see graphic below) travel from the mitochondria, across the cell and into the nucleus of that cell, presumably to synthesize requisite molecules for growth. (Some years ago, I wrote about the traveling PDH enzyme research, here but had not considered its impact on cell fate more generally).
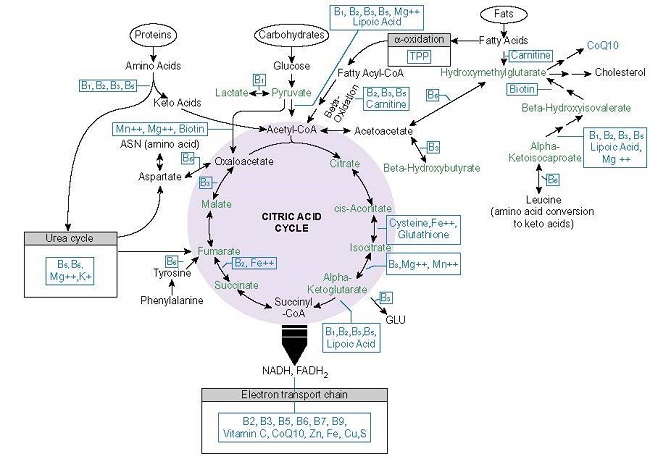
Nutrient Status Drives Metabolism
From this sampling of the research, it is clear that metabolism, which boils down to the nutrient status and energetic capacity of the mitochondria, determines cell fate, and although genetic instructions and epigenetic molecules are important, those instructions cannot be executed without the appropriate metabolic capacity. When a cell is faced with a decision about whether to live or die, reproduce or set up defense protocols, it tests the environment before taking action. Those tests determine outcome.
The cell tests whether it has the materials in its environment. If it cannot execute the metabolism, then it won’t become that cell type, in spite of signals to differentiate.
So, even though genetics and epigenetics are telling the cell to execute a particular plan of action that plan is overridden by the mitochondria within that cell if the environment is unfavorable. When this is the case, cell fate decisions focus on defensive measures. Here, we can see how cancer and other disease processes not only represent the result poor metabolic capacity relative to environmental demands, but at their foundation are simply mitochondrially-induced defense mechanisms. Indeed, with cancer in particular, researchers found that when tumor cells are placed in an environment with unhealthy mitochondria they thrive and grow, but when healthy mitochondria are present, they don’t. Taking this a step further, defects in mitochondrial metabolism have been shown to expedite the aging and senescence of cells by accelerating telomere erosion and epigenetic damage and promote genome instability and oncogenesis. In other words, poor mitochondrial function initiates the very epigenetic and genetic defects expressed in cancer and other disease processes.
In this regard, the metabolic environment becomes the most important element in development and in health or illness. Environment, in its totality, is not an ancillary tuning fork for genetic or epigenetic programming and not something to be cursorily addressed or allocated to the dustbin of randomness. It is everything.
‘Instead of thinking about the gene expression networks just happening to interact with metabolism, it’s really metabolism driving [developmental decision-making],’ he said, ‘and gene expression networks are the tools by which that occurs’.
If this research tells us anything, it is that we are not hardwired, immutable, and largely, impenetrable machines that just happen to suffer developmental anomalies or fall ill to random genetic aberrations of the cancerous type. Rather, we are energetic beings interacting with the environment. The seat of that energetic capacity rests with the mitochondria. Mitochondria are key to everything, and so, if we tend to our mitochondria, and more broadly, to our environment, something many of us are loathe to address honestly, the chances of random acts of cancer and other chronic illnesses are reduced. It also means, the reproductive capacity and outcomes are improved.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Feature image created in Canva AI.