Over the last few years, it has become increasingly apparent how important thiamine is to overall health. Thiamine (thiamin) or vitamin B1, sits atop the mitochondria at multiple entry points involved in the metabolism of foods into cellular energy (ATP). It is also critical for several enzymatic reactions within the mitochondria. We have illustrated repeatedly how thiamine deficiency leads to mitochondrial dysfunction, which in turn leads to complex multi-organ system illnesses characterized by chronic inflammation, disturbed immune function, altered steroidogenesis. Each of these is related to deficient mitochondrial energetics. When serious or chronic, thiamine deficiency leads to erratic autonomic function, now called dysautonomia, and a set of disease processes called beriberi.
Long before those symptoms emerge and absent severe deficiency, marginal thiamine status evokes subtle changes in metabolic function. Among these changes, enzymes that would normally metabolize certain foods fully and into useful substrates for other functions are downregulated, shifting the metabolic pathway towards more toxic end-products. The chemistry is complicated and we will go over it in a moment, but first I would like to propose a framework for understanding metabolism. For me, it is useful to imagine metabolism visually as giant maze of right and left turns; where wrong turns lead to dead ends and dead ends lead to the build up of endogenous toxins. Among the primary variables determining the route metabolism takes is enzyme nutrition.
Enzymes require nutrient cofactors to perform their metabolic tasks. When the appropriate nutrient co-factors are present in sufficient concentrations for the enzymes to operate fully, the food we eat is successfully metabolized into end-products that are useful for all manner of processes and cellular energy is produced. Even in the case of genetic aberrations that limit enzyme function endogenously, there is evidence that nutrient manipulation can overcome inadequate enzyme activity. When nutrient co-factors are in short-supply, however, resources are reallocated. Metabolism shifts directions, it takes a right turn when it should move left or vice versa. Different enzymes are activated and metabolism eventually reaches a dead end but not before potentially toxic, unused waste products build up. As these toxins build up, other systems become disrupted, inflammatory and immune responses are activated, demanding ever more energy to resolve. It is this energy spiral, I believe, that induces and maintains many of the illnesses we see today. This means that observing how one reacts to certain foods may point us to correctable nutrient deficiencies.
The Rise in Food Sensitivities
In recent years, I have become fascinated by the growing preponderance of food sensitivities and intolerances. It seems everyone has a problem with something. Given the current practices used in industrial food production, I suppose it is no wonder. We use a staggering number of chemicals to grow and process foods; chemicals that reduce the nutrient content of supposedly healthy foods, but also, present as toxicants that must be dealt with metabolically when ingested. The double hit of low nutrients/high toxicants is disastrous for metabolism. Throw in the generally high calorie content of the western diet and one has to wonder how our mitochondria function at all. And yet they do. Well, sort of. If we don’t count the exponential growth in chronic and seemingly intractable illnesses, but I digress. I believe that food, or lack of quality food, is top among the core contributors to modern illness and food sensitivities are among the key early warning signs of poor metabolism and by definition, faltering mitochondria.
Oxalate Problems
One of the more intriguing and troubling food intolerances that has become increasingly common is to the high oxalate foods. Oxalates are natural substances found in many healthy foods, especially dark leafy greens like spinach, that bind calcium and other minerals, and when left unmetabolized, can form crystals leading to kidney stones. Approximately 10% of men and 7% of women experience at least one episode of kidney stones across the lifetime. Beyond the kidney stone, oxalate intolerance is linked to wide range of chronic health conditions largely due to the build up oxalic acid which may or may not bind calcium, but causes problems nevertheless. Poor oxalate metabolism disrupts gut health, shifting the microbiome unfavorably causing dysbiosis, damages the mitochondria and induces system wide oxidative stress, inflammation and immune reactivity. Problems with oxalate metabolism have been found in individuals with autism, multiple sclerosis, arthritis, and fibromyalgia to name but a few. A common and usually somewhat successful remedy is to avoid the consumption of high oxalate foods. Below are some of the more common high oxalates.
Figure 1. High Oxalate Foods

Absent genetic aberrations leading to poor oxalate handling, I cannot help but wondering if the avoidance diet is the correct response, especially permanently. Certainly, it would help short term, and there may be foods that result in oxalate accumulation that could or should be avoided long term, but an across-the-board and permanent avoidance of most oxalate producing foods seems problematic nutritionally. If we consider that many who suffer from oxalate issues may also be sensitive to other foods, the avoidance approach could limit dietary options considerably. What if we are approaching this issue incorrectly? My gut tells me, and research seems to back it up, that barring genetic issues with oxalate metabolism, the dietary component is not simply one of excess oxalate consumption. It is a problem with inadequate nutrient consumption in the face of excessive non-nutrient foods – e.g. it is a problem with the modern western diet in its entirety.
Other Dietary Contributors to Oxalate Buildup: Processed Foods
If we dig into the oxalate issue a little more, we see that foods resulting in excess oxalate storage are not necessarily limited to whole foods listed above in Figure 1. A number of foods classified as high oxalate, are simply processed food products, high in carbohydrates, trans fats and low in nutrients. Below is a graph of some of the higher oxalate foods as compiled by the University of Chicago via Harvard’s School of Public Health. Notice, how processed foods make this list. Sure, their oxalate status is significantly lower than other foods, but consider what portion of the average western diet these products comprise. Click the links above to see a more complete listing foods that result in high oxalate accumulation. When you search through those lists (especially, the one from the University of Chicago), it becomes apparent that virtually all processed foods can result in oxalate problems.
Figure 2. Oxalate Content in Common Foods
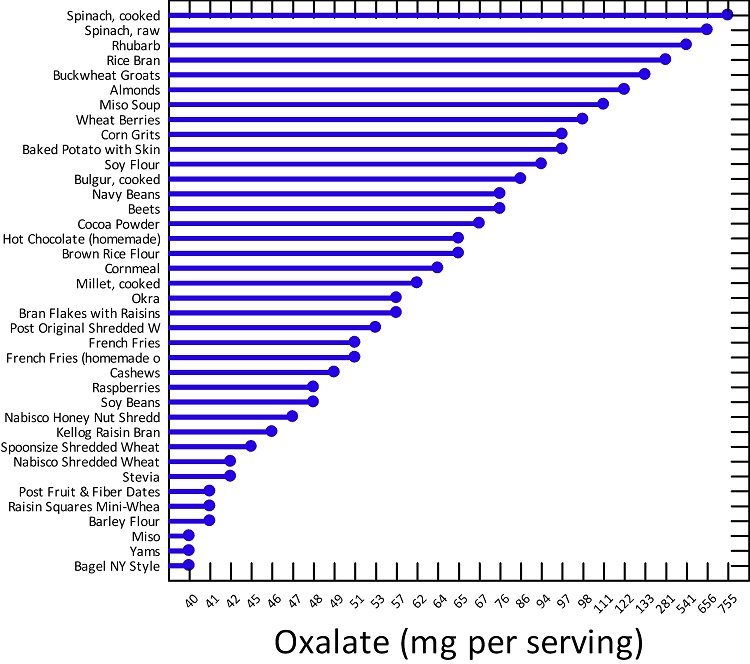
One could argue that oxalate buildup involves shifts in the metabolic pathway that are directly related to nutrient deficiencies and those nutrient deficiencies emerge from the consumption of the modern diet. Processed carbohydrates, for example, in the presence of thiamine deficiency, are metabolized quite differently than when thiamine is present, with the former resulting in oxalate build up. Since a diet high in processed carbohydrates is one of the leading causes of thiamine deficiency in the first place, this begs the question, is the issue really oxalates or a sort of high calorie malnutrition resulting in thiamine deficiency, where oxalate accumulation is just a side-effect. Similarly, when thiamine is absent, fatty acid metabolism can go awry, making highly processed, high carbohydrate, high fat foods damaging on two fronts.
Finally, there are many other foods that can lead to high oxalate production in the presence of low thiamine including: beer, wine, tea, coffee, yogurt, bread, rice, soybean paste, soy sauce, and oil, along with foods that have been fermented, roasted, baked, or fried. And just like high carbohydrate diets can lead to thiamine deficiency, as nature would have it, all alcoholic drinks, coffee, and tea decrease thiamine uptake thereby both creating and exacerbating the thiamine deficiency that leads to oxalate accumulation. It could be that problems with oxalates is simply the early sign of thiamine deficiency and it may very well be a protective mechanism of sorts, a metabolic diversion, albeit an unhealthy one, to forestall the other issues associated with insufficient thiamine intake.
I should also mention that oxalate problems may not be solely related to diet. Inasmuch as all pharmaceuticals damage the mitochondria and either decrease thiamine directly or increase the demand for the need for thiamine indirectly, regular use of pharmaceuticals may also contribute to the problem. Similarly, a number of environmental exposures increase glyoxal (a precursor to oxalate build up in the face of low thiamine), including: cigarette smoke, smoke from residential log fires, vehicle exhaust, smog, fog, and some household cleaning products.
Is Thiamine Really the Problem?
It may be. The chemistry is complicated and detailed below, but basically, marginal thiamine status, prevents the proper metabolism of certain foods leading to the build up of toxins while simultaneously crippling the natural detox pathways. The combination of increased toxins and decreased detox ability leads to all sorts of damage and illness, high oxidative stress, and as illustrated by the graphic below, can lead to cancer (to be discussed in a subsequent article). Thiamine prevents this. A paper published in 2005, (from which Figure 3., is taken) details just how many mechanisms that lead to oxalate accumulation are initiated by low thiamine.
Figure 3. How Low Thiamine Leads to Elevated Glyoxal and Cancer
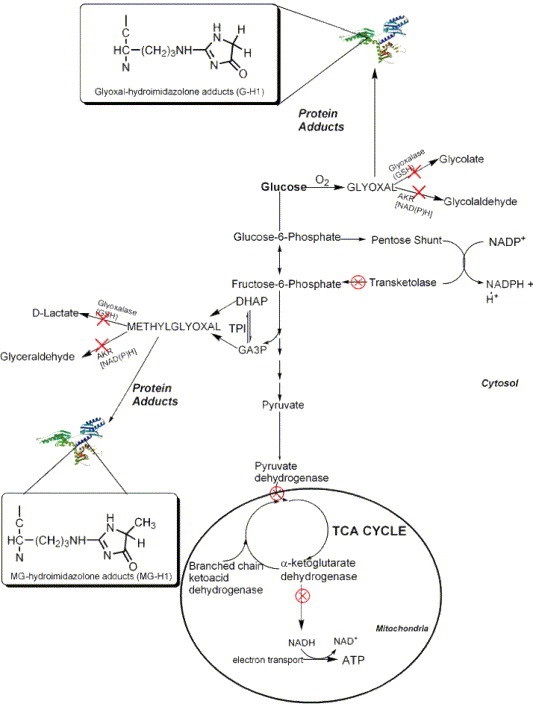
The specifics involve a metabolic diversion that leads ‘food’ metabolites down what is called the glyoxal pathway, the pathway responsible for oxalates. Each of the red ‘X’s’ indicates an impaired thiamine dependent enzyme.
With Marginal Thiamine
- Elevated glyoxal and methylglyoxal
- Diminished activity of thiamine dependent enzymes (transketolase, pyruvate dehydrogenase, branched chain ketoacid dehydrogenase, and a-ketoglutarate)
- Low transketolase = low NADPH
- Low NADPH = low glutathione (the primary detoxification agent in the body; glutathione also requires vitamin C)
- Low glutathione = poor detoxification of glyoxal and methylglyoxal = increased carcinogenic protein adducts
- High Oxalate Foods
- Diminished activity of thiamine dependent enzymes (transketolase, pyruvate dehydrogenase, branched chain ketoacid dehydrogenase, and a-ketoglutarate)
- Diminished pyridoxal kinase (PK) activity*
- *This is not discussed in the aforementioned paper, but should be included. PK is the enzyme that converts the inactive form of vitamin B6 (pyridoxine 5-phosphate) into its active form, pyridoxal 5-phosphate (P5P). Low P5P prevents glyoxalate from being converted back into glycine, leading to high oxalates. Many mistakenly assume that low vitamin B6 is responsible for high oxalates. While that is possible, it is also possible, and often more likely, that low thiamine is responsible.
With Sufficient Thiamine
- Thiamine dependent enzymes work appropriately
- Sufficient transketolase activity = sufficient NADPH
- Glyoxal and methylglyoxal are metabolized into other substrates and/or excess is detoxified
- Sufficient NADPH = sufficient glutathione
- Glyoxalate is converted to a-hydroxy-b-ketoadipate or glycine and not oxalate
- Alanine glyoxylate amino transferase (AGT), the enzyme required to convert glyoxalate into to glycine instead of oxalate has sufficient activated vitamin B6.
This is not to say that there are not other vitamin and mineral deficiencies also associated with hyperoxaluria, there are. Research has shown that low magnesium (a requisite co-factor in many of same enzymes as thiamine), along with low vitamin A, in addition to the low vitamin B6, mentioned above play a role. Vitamin E may also be involved.
Take Home
The majority of modern illnesses are the result of poor diet and environmental exposures directly, cumulatively, and generationally. Over the span of a few short generations, we have forgotten that food is fuel and that good, clean, unprocessed food is required for health. The allure of processed foods and cheap agriculture through chemistry, has left much of the population starved of nutrients, while simultaneously bearing a high toxicant load. The result is all sorts of metabolic disturbances which may manifest as food insensitivities and intolerances. It is interesting to note that the metabolic changes involved in oxalate buildup do not require what we would consider a full-scale thiamine deficiency, but rather, a sort of thiamine insufficiency initiated by marginal thiamine intake, something that is likely common across populations.
A less complicated overview of the low thiamine > high oxalate connection can be viewed below.
Is Your Body Producing too much Oxalate?
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image: Scanning electron micrograph of the surface of a kidney stone showing tetragonal crystals of weddellite (calcium oxalate dihydrate) emerging from the amorphous central part of the stone; the horizontal length of the picture represents 0.5 mm of the figured original. Image credit: Kempf EK – Own work, CC BY-SA 3.0.
This article was published originally on August 15, 2019.















NOTHING mitigates oxalates they can’t be soaked, sprouted, baked, boiled or pressure cooked to safe levels. Supplements of calcium and magnesium citrate can reduce oxalate absorption from the intestine. The only way to be safe from them is not to eat them. Taurine MAY help stimulate bile salt production (taurocholic acid), leading to better fatty acid absorption and diminished oxalate absorption. Supplements help because oxalates damage everything they touch. Once you’ve been oxalate poisoned you don’t fool around – you throw out your juicer and never buy spinach again. Then you start to heal following the Trying Low Oxalate protocol.
I have been able to slowly reintroduce certain oxalate-y foods without issues, so praise be! My sleep is the biggest issue i face, it hangs on such a thin thread. I can sleep fine after eating some potatoes or even sourdough bread, but 1 thing i absolutely still cannot consume even a drop of is canola oil/vegetable oils. They aggravate my liver just as bad as alcohol and it keeps me up aaaaalll night!
I can’t eat anything with oxalates since taking a course of the antibiotics “ Flagyl”. I don’t see anyone talking about the fact that not having the microbe in your gut called “ Oxalobactor Formigenes “ is what causes most people to have problems with oxalates degradation. There’s no way to repopulate my gut with this beneficial microbe now that the colony has been destroyed from the antibiotic I took. I’m going to do my own “ Fecal Transplant “ like clinics do for C.Diff. Infections. It’s the only thing that will help. I’ve been suffering for 8 years now. I can only eat 3 things.
Oxalate is an endpoint of normal metabolism and has no function in the body. It is an ash resulting from oxidation. An accumulation of excess ash is always an indicator of inefficient combustion and oxaluria in some people may indicate poor nutrition or a gene defect in energy metabolism. That is why it is associated with thiamine because thiamine stimulates energy production.
So one should not focus on avoiding oxalate in diet, but rather focus on fixing energy production with thiamine and friends?
Perhaps both.
Hi Susan
Could you point to papers or info on the way oxalates change the trafficking of iodine (and sulfates). Many thanks.
Hi Sue,
Did you ever manage to find anything on the way oxalates change the trafficking of iodine and and sulfates ?
Thank you for this informative article. I’ve been doing my own research, and it seems that deficiencies of vitamins A, B1, B6 and E as well as alpha lipoic acid can encourage oxalates to have a problem, and that taking them can be very helpful.
Additionally, studies have shown that hydroxycitrate, as found in Super CitriMax dericed from garcinia cambogia is most effective in dealing with oxalates in the intestines.
I share your concerns about being on a limited oxalate diet for years, or carnivore, as some are doing. That could result in other non oxalate health problems. The low oxalate community seems to have blinders on, so focused on just autism. Their dogmatic use of the OAT test leaves them vulberable to nutrient deficiencies/imbalances not tested by OAT.
In the end, being aware of one’s own genetics and environmental factors and nutrient status is wise, as is customizing a program that’s personalized for each patient.
Summarized from David Shaw, PhD
1. Oxalates are primarily from 3 sources: the diet, antibiotics and possibly candida as well as human metabolism.
2. Use antifungal drugs to reduce yeast and fungi that may be causing oxalate.
3. Calcium citrate reduces oxalate absorption in the intestine. Take with meals.
4. Vitamin b6 is a cofactor for one of the enzymes that degrade oxalate
5. Increase water intake to eliminate oxalates.
6. Lactobacillus Acidophilus and Bifidobacterium have enzymes that degrade oxalates.
7. High amounts of Omega-6 fatty acid is associated with oxalate problems
8. Take supplements of vitamin E, selenium and arginine to reduce oxalate damage
9. Vitamin C can break down to form oxalates if exceed 4 g day.
10. Genetic hyperoxaluria type 1 is associated with vitamin B-6 deficiency
11. High oxalates may form in the blood if exposed to inorganic mercury (from vaccinations or antibiotics)
12. Magnesium oxalates the more soluble than calcium oxalates but oxalates from food/yeast/fungal sources are more likely to be absorbed with magnesium. Magnesium from Epsom salt baths might help dissolve calcium or mercury oxalates
Link: https://www.greatplainslaboratory.com/articles-1/2015/11/13/oxalates-control-is-a-major-new-factor-in-autism-therapy
Give supplements of calcium citrate to reduce oxalate absorption from the intestine. Citrate is the preferred calcium form to reduce oxalate because citrate also inhibits oxalate absorption from the intestinal tract. The best way to administer calcium citrate would be to give it with each meal. Children over the age of 2 need about 1000 mg of calcium per day. Of course, calcium supplementation may need to be increased if the child is on a milk-free diet. The most serious error in adopting the gluten-free, casein-free diet is the failure to adequately supplement with calcium.
Source; Oxalates Control is a Major New Factor in Autism Therapy
Nov. 16, 2015
Great Plains Laboratory
William Shaw, PhD
There are others ways as well to bring down oxalates. A person’s individual tolerances (genetic mutations/alterations) can cause problems with this protocol. Perhaps for many this is helpful, but not all. Hence, more digging into why. Healing our bodies is not a one-size-fits all.
Chandler,
I would like to say that our group, Trying Low Oxalates, started after the science on oxalate discovered that oxalate moves all over the body once it is in blood. Oxalate’s presence will change the trafficking of sulfate and iodine, and the regulation of pH everywhere in the body where the SLCa6A family of transporters is found. These are exchangers, so they are balancing out the levels of a defined list of substrates on opposite sides of a membrane.
The thyroid gland has been shown to collect oxalate from birth, and that oxalate, if it builds up, often may lead to thyroid dysfunction.
If you want to get a feel for what oxalate might affect, please have your readers look at diseases in the literature where elevated oxalate has been found for decades. That would include cystic fibrosis, where problems with the CFTR lead to elevated oxalate. This genetic disease leads to issues in the lung and GI tract. The CFTR regulates the SLC26A family of transporters, and therefore the movement in the body of oxalate and sulfate, and better known, chloride.
Also consider other diseases of elevated oxalate that have long been in the literature like Down syndrome and inflammatory bowel diseases and even celiac sprue.
TLO started fifteen years ago and has been data driven. We found out with a team of scientists in Poland that oxalate is extremely elevated in autism and that work was published in the European Journal of Paediatric Neurology.
We have people test their urine to find out their oxalic acid levels there. If oxalate is elevated, then the composition of their previous diet can tell them if oxalate might have come from that source. They can see whether oxalic acid levels come down after they begin reducing dietary oxalate. We only advocate a slow and gradual reduction while maintaining nutritition.
Those concerned about nutrition on the low oxalate diet should read this article that one of our moderators wrote for the Journal of Gluten Sensitivity. https://www.celiac.com/articles.html/journal-of-gluten-sensitivity/journal-of-gluten-sensitivity-spring-2016-issue/get-your-super-foods-eating-high-nutrition-without-the-oxalate-r3706/
Oxalic levels show up in urine from food after its oxalate was already absorbed into the blood. Once in urine, we know diet sourced oxalate already got past protections in the gut.
We also look at the markers glycolic and glyceric which have more to do with the oxalate that our bodies make, often from vitamin issues, or metabolic errors, or even issues in sugar metabolism.
The body doesn’t care where the oxalate came from. All sources are additive and equally toxic when high enough.
Your readers should know that thiamin issues generally do not raise urinary oxalate because thiamin’s issues are more intracellular. Thiamin issues may raise urine glyoxylate (which is generally not measured). This source of oxalate, because of its intracellular location, is much more likely to lead to problems with biotin dependent carboxylases. Four out of five of those enzymes make their home in the mitochondrion.
Unfortunately, these carboxylases are not the only set of mitochondrial enzymes oxalate is known to impair. Scientists consider mitochondrial dysfunction to be a primary consequence when there is elevated oxalate in tissues.
Chandler, please encourage your readers to be data driven. If oxalic acid is not elevated in urine the first time, then test it again if you know your diet is high in oxalate. Some oxalate stores and there seem to be rhythms to oxalate secretion, and if ratioed to creatinine, normal levels that scientists have found are much lower than we find now with typical lab reference ranges.
I will write more later, but I must sleep!
Thank you Susan. Your answer is spot on.
Blessings
Thank you so much, Susan, for all you do for people like myself with oxalate issues.
Hi Susan
Could you point to papers or info on the way oxalates change the trafficking of iodine (and sulfates). Many thanks.
As a person who has High Oxalate issues I disagree with your argument that avoiding those items long term could be problematic. I will use kale as an example. For those who can eat kale there are several forms that are Low Oxalate – Dino [AKA lacinato, tuscan kale, cavolo nero, etc] is Low Oxalate as is Russian kale, With kale it’s a simple matter of changing out the variety that one has been eating and switching to that low oxalate kale.
With regards to potatoes – potatoes are inflammatory vegetables. For those who have arthritis and/or other inflammation issues, potatoes really should be avoided. All is not lost with regards to potatoes. For those who absolutely cannot begin to think of giving them up – the small new, red potatoes are Low Oxalate. One can make home made french fries with them as well as potato salad and mashed potatoes.
Instead of sweet potatoes – switch to butternut squash. For me, butternut squash – and all the other squashes – are my new BFF’s.
I don’t lack for nutrition on the Low Oxalate diet. In fact, nutritionally, I think I have MORE nutrition daily than I had when I ate all those High Oxalate foods that I no longer eat. How can one have nutrition if the High Oxalate foods cause one to be sick all the time and constantly throw up? Throwing up is NOT healthy.
Annie, have you considered taking specific strains of probiotics that are proven to reduce and even eliminate oxalate? There are quite a few strains that do the job:
https://aem.asm.org/content/76/16/5609.full
Do you have a reference for red potatoes being low oxalate? I can’t find info regarding this. The closest I have found is they may be medium oxalate.
Try Low Oxalate group has testing information available to members. Only the “small” red potatoes are “low” oxalate. The medium and larger potatoes have a higher concentration of oxalate as they grow and mature.
Thank you Dr. Marrs for the article on oxalates. A government website indicates that supplemental taurine may mitigate oxalates. Any thoughts?
Wayne, I do not know, but the folks at the Trying Low Oxalates group on Facebook, may have the answer you are looking for.
I do believe from research that low thiamine is often at the root of many of these metabolic disturbances.