The pollution we release into the environment affects all of us, not least of all the delicate ecosystem of gut microbes that keep us healthy. The gut microbiome plays a pivotal role in our health – from energy production and immune function, to cognitive development and homeostasis. In fact the metabolic activities of our gut microbes are so vital to health that the gut is now considered an organ in its own right. It should be no surprise then that the hundreds of environmental pollutants our bodies are exposed to every day have profound adverse effects on our microbiome.
This article focuses on three categories of environmental pollutants and their effects on our microbiome – air pollutants, pesticides and heavy metals.
Air Pollution Linked to Obesity and Diabetes
Air pollution is the fourth leading cause of death worldwide. The particulate matter in air pollution can alter the gut’s composition and function by either inhibiting or promoting the growth of certain microbes.
Air pollution greatly influences the rate of obesity and type 2 diabetes. In fact, the geographic distribution of diabetes globally correlates with air pollution. In 2016 alone, air pollution (particularly PM2.5) contributed to 3.2 million cases of diabetes globally. The mechanism by which air pollution drives these diseases is through particulate matter altering the gut microbiome and its metabolic activities as illustrated:
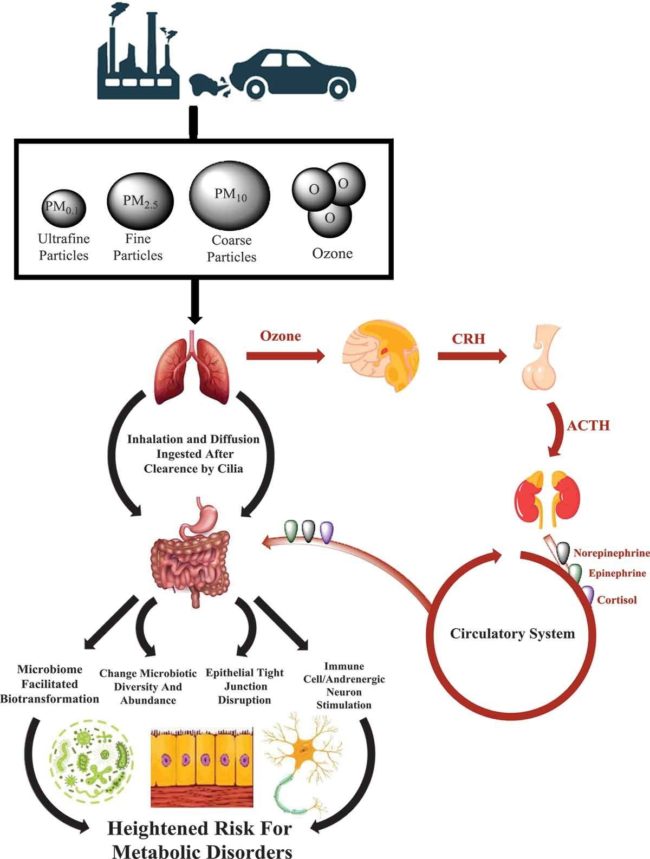
Reference: Bailey et al, Exposure to air pollutants and the gut microbiota: a potential link between exposure, obesity, and type 2 diabetes, 29 April 2020.
A number of studies demonstrate elevated levels of air pollution exposure are associated with several gastrointestinal diseases, including inflammatory bowel disease (IBD), irritable bowel syndrome, appendicitis, and GI disorders in infants. Cigarette smoke, which contains particulate matter that is also present in air pollution, has also been shown to alter gut composition in human and animal studies.
A 2020 study of 101 young Southern Californians (averaging 19.6 years old) found that exposure to air pollutants was associated with alterations in gut bacterial diversity. The variation in diversity was significant with 4.0% variation for total Nitrogen Oxide (NOx) exposure, 4.4% for Nitrogen Dioxide and 11.2% for Ozone. This study also found that higher ozone exposure influenced gene pathways involved in fatty acid synthesis/degradation, all of which may play a role in gut barrier integrity, obesity and Type 2 diabetes.
Another study of young people (aged 17-19) in Southern California, found that increased exposure to nitrogen oxide pollution near roadways influenced the abundance of gut bacteria (Bacteroidaceae and Coriobacteriaceae) that have been associated with obesity and altered metabolism.
In a 2013 study of mice exposed to PM10 (very small particulates found in dust and smoke), for just 7-14 days, researchers found that the pollution altered the mice’s immune gene expression, enhanced pro-inflammatory cytokine secretion in the small intestine, increased gut permeability, and reduced white blood cell activity. When this exposure was increased to 35 days, even more inflammation in the colon was observed together with altered short chain fatty acid concentrations and changes in microbial composition.
A 2019 epidemiological study in China found that gut microbes (particularly Firmicutes, Proteobacteria and Verrucomicrobia bacteria) play an important role in mediating the effects of particulate matter when it enters the body. However, both PM2.5 and PM1 reduce gut microbial diversity, in turn increasing the risks of developing Type 2 diabetes.
In summary, it is clear that air pollution negatively alters gut bacteria, setting the scene for increased disease risk in animals and humans, particularly Type 2 diabetes and obesity. Efforts to address the global obesity epidemic therefore should not overlook the role of our environment in driving disease, together with lifestyle and psycho-social factors.
Pesticides Affect Gut Bacteria
Several studies demonstrate that gut microbiota help protect us against the toxicity of pesticides but once overwhelmed, disease can set in. For example, ingesting the broad-spectrum pesticide chlorpyrifos alters the gut microbiota of mice, contributing to obesity and insulin resistance.
Some people (largely those connected to chemical companies) argue that certain pesticides are not harmful to humans because the pathways that they target do not exist in the human body. This is the argument made for glyphosate (RoundUp) in relation to the shikimate pathway. However this ignores the fact that many of these pathways do exist in the microbes living in our gut, thereby explaining the harmful effects that pesticides can have on our health.
For example, herbicides like 2,4-D (used for lawn and weed control), may affect gut bacteria because not only plants but also bacteria can synthesize plant hormones. Both pure glyphosate and glyphosate-based formulations alter the bacterial makeup of the gut microbiome in rodents and honeybees.
Further, the fungicide imazalil (used as a preservative and to prevent decay and control fungal infections in fruits and vegetables) induces microbiota dysbiosis and hepatic metabolism disorder in zebrafish and causing adverse alterations to the microbiota of mice.
Another study showed that the insecticide diazinon damaged the microbiomes and metabolic profiles of mice. Interestingly, exposure to diazinon and another insecticide called malathion influences the ability of gut bacteria to modulate gene expression (through a process called quorum sensing), yet another sign of the ways in which pollutants can drive disease through alterations to gut microbiota.
Whilst there are fewer studies examining the effects on the gut of agro chemicals beyond glyphosate and chlorpyrifos, studies in adults across multiple species using a wide range of agro chemicals find gut microbiome alterations as well as impaired lipid metabolism, oxidative stress, and inflammation among other things.
The Heavy Metals Toxins
Heavy metals are elements found in the Earth’s crust which are highly toxic even at low concentrations. Gut bacteria play an important role in metabolizing and eliminating heavy metals from the body. For example, human gut bacteria can transform inorganic arsenic into less toxic organic arsenic, whereas demethylation of methyl mercury by gut bacteria creates more toxic inorganic mercury.
A 2014 study of mice exposed to arsenic in drinking water for four weeks, found disturbed gut microbiota and metabolic activities. A 2017 study of mice found similar results from lead exposure, with vitamin E, bile acid, and nitrogen metabolism all impaired. Similar results were found in a 2018 study that exposed rats to a range of heavy metals including arsenic, cadmium, cobalt, chromium, and nickel. In a 2019 study of frogs, cadmium exposure also altered gut microbiota, and so too with fish.
A 2018 study of fish (fathead minnows) and mice exposed to mercury found that it damaged their guts and impaired lipid metabolism and neurotransmission. Nutritional supplementation may help to soften these effects however, with selenium reversing some of the gut damage in mercury-poisoned rats.
Like mercury, studies show that magnesium chloride damages the gastrointestinal tracts of mice and chickens, causing thicker muscle walls, wider submucosa, a decrease in goblet cells and necrosis in gut enterocytes. In chickens, magnesium chloride exposure also adversely altered the metabolism of xenobiotics (any substance that is foreign to animal life), and PAHs (chemicals found in coal, oil and gas).
A 2018 study found that protein rich diets can alter the microbiome, in turn assisting with mercury elimination, in humans. A 2019 study of adult Bangladeshis reported that arsenic exposure altered the gut microbiome and resulted in an overproduction of the Citrobacter bacteria, which can cause a range of health problems including urinary tract infections, respiratory diseases, gastrointestinal inflammation, and sepsis in immunocompromised individuals. Citrobacter is also associated with higher vascular intima-media thickness (IMT), a subclinical marker of atherosclerosis. As such, arsenic exposure may play a key role in the development of atherosclerosis.
A Fundamental Rethink Is Required
In conclusion, it is clear that pollutants profoundly influence the gut bacteria of humans and animals, impairing healthy metabolism and therefore increasing the risk of disease. Just as the pollution we create is damaging our environment, so too it is damaging us, yet another sign that we are more intimately connected with the environment than our extractivist economies acknowledge. Whilst there are many steps we can take as individuals to protect ourselves against environmental pollution, such as eating organic/chemical-free nutrient-dense whole foods, exercising, reducing stress, minimizing our exposure to known pollutants, and supplementing to strengthen our immune systems, ultimately it is the systems that drive this pollution which need to change. That requires a fundamental rethink of our politics and economies so that they no longer treat the earth and us as expendable resources to fuel unfettered growth.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Image by Foto-Rabe from Pixabay.
This article was published originally on May 3, 2021.















I have no doubt that any reader of this post would agree. The issue, however, is what can we do about it as a cause of poor health? We are not going to stop pollution! The answer is in Charli’s post——nutrition! Unfortunately, the description of the cause and the treatment both fall on deaf ears. Neither the long suffering patients, nor the physicians to whom they turn, accept nutrition as a defense. The point is that the nutrition we need for supplying the energy to survive in a polluted world is ignored. Most people are quite ready to take any number of pills as medicine. Perhaps we might persuade them that micronutrients are the missing item in our excess of artificial foods and that vitamin enrichment is not sufficient to meet the prolonged deficiency that is so common. Often diagnosed as psychosomatic in the present medical environment (“the lab tests are all normal”), perhaps we can offer micronutrient pills to catch up with the empty calories!!