That the brain demands glucose and mostly, if not only, glucose has been ingrained in medical thought for generations. Although, ketones are recognized as a secondary and presumed backup fuel source when glucose is absent, neither the fatty acids that produce the ketones as a byproduct of beta-oxidation, nor the amino acids from proteins are considered relevant to brain energy – at least not in conventional texts. Given that the mitochondria in body use all three nutrient pathways, carbohydrate, fat, and protein, to produce energy, it has always struck me as, not only odd, but suspiciously inefficient and fundamentally unsafe that such a complex and important organ as the brain would use only or predominantly one fuel source. It turns out, our long held assumptions about the brain may not be accurate. Like the rest of the body, it does indeed utilize all fuel sources. Also like the rest of the body, different regions of the brain and cell types, have different fuel source preferences. Brain fuel, it appears, does not just come from glucose.
Food Is Energy
Before we discuss brain fuel, let us talk about how the foods we consume become energy. It is important to understand, that all cells need energy to do things they have to do. No energy, no cell function, no life. Most of that energy comes from organelles within the cells called mitochondria. Mitochondria take components of the foods we eat, process it, and spit out energy in the form of ATP molecules. Figure 1. below illustrates the macronutrient pathways and the micronutrient-dependent enzymes involved in ATP production.
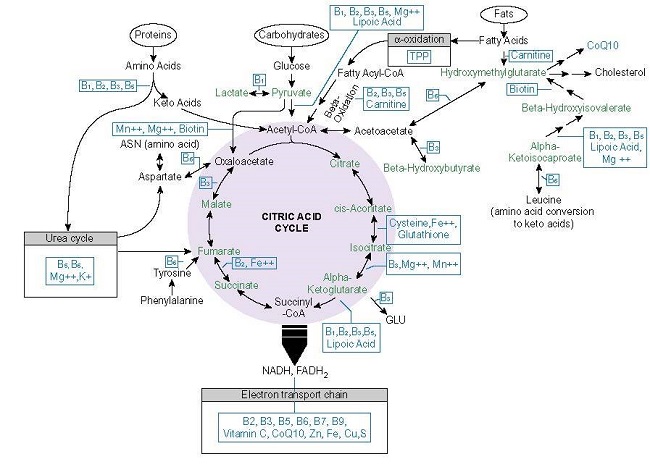
Although the details of that production process are complicated, the basics are simple. Consumed foods are metabolized by three primary pathways (and a whole bunch of ancillary pathways) in the cell; one for glucose, one for fatty acids, and one for proteins. Each ultimately leads into the mitochondria where, when combined with oxygen and given sufficient micronutrients (vitamins, minerals, and metals), mitochondria will spit out a certain number of ATP molecules. How much ATP depends upon the food substrate, micronutrient availability and other variables. From an article I wrote a few years ago:
From the oxidation of one glucose molecule, we get around ~30-36 ATP molecules. From one fatty acid molecule, we get over 100 ATP molecules, and from the amino acids, we get either substrates for the synthesis of other proteins, more glucose to metabolize and feed into the mitochondria (amino acids can be converted into glucose via gluconeogenesis), or another compound called pyruvate that may also be used by the mitochondria to make fuel or converted into lactate.
When things are not going as well or when quick energy is needed for certain functions, a number of extra-mitochondrial pathways, are used to break down the various macronutrient components to provide energy. There are two key pathways here. One is called glycolysis and the other is called the pentose phosphate pathway (PPP), which connects glycolysis with the TCA [tricarboxylic acid also known as the Krebs of citric acid cycles] and OXPHOS [oxidative phosphorylation] and performs a few other important tasks like providing substrates for DNA and RNA synthesis. The PPP nets 1 ATP molecule per molecule of glucose, while glycolysis nets about 2 ATP molecules per molecule of glucose. A third pathway, used primarily in quickly replicating immune cells and cancer cells, involves shuttling glutamine, an amino acid, through the side door of the mitochondria to produce ~24 units of ATP per unit of glutamine. Severely stressed neurons use this pathway as well.
Which pathway is used to produce ATP may alternate according to need, cell type, fuel type, micronutrient, and/or oxygen availability. Some cells metabolize a good portion of their energy in the cytosol using glycolysis while others rely almost exclusively on the OXPHOS in the mitochondria. This flexibility allows cells to adapt rapidly to changing circumstances. If one macronutrient is not available, energy is derived from another. When a micronutrient (vitamin or mineral) is not available, the products are diverted through other pathways, and when there is limited oxygen, glycolytic pathways will take over. All of this is meant to help the body metabolize different compounds and maintain energy homeostasis relative to environmental demands.
There a few things about the energy production process that I would like to point out. First, fat molecules produce almost triple the ATP units than glucose. This is one of the reasons I find it suspect that cells in the brain are not taking advantage of this substrate for fuel. Second, the amino acids from proteins are important energy pathway as well. Yes, they are used for other functions, but they can be used for fuel as well. Third, mitochondria, via OXPHOS, are responsible for the bulk of the energy a cell uses – about 90%. This is an oxygen intensive process. The oft repeated statement that brain mass comprises only 2% of total body mass but consumes 20% of the oxygen originates from this fact. Finally, although extra-mitochondrial energy production occurs in all cells, it is more limited than what can be produced within the mitochondria. This holds true for the brain cells as well.
That said, cell populations have preferences. Cardiomyocytes and nephrocytes prefer fatty acids over glucose; muscles change fuel preferences based exertion; immune cells like the amino acid glutamine and so on. Despite preferences for a particular fuel type, cells will take what they need from what is available. If only certain types of foods or particular macronutrients are available, the body will adapt and survive. It may not be healthy or fully functional, but it will survive, for as long as can. The brain is no different. It will use what it has available to survive.
Ketones and Lactate
Before we discuss the use fatty acids or proteins for fuel, it is important to recognize that ketones, molecules produced by the liver and by brain astrocytes, when carbohydrates are low, are a well-known ancillary brain fuel. Inducing ketogenesis by restricting carbohydrates has been used clinically to manage epilepsy (since the 1930s), migraine (for a decade or so), and more recently has gained popularity as a treatment for a range of psychiatric conditions. In the absence of dietary glucose, ketones (acetoacetate and b-hydroxybutyrate), derived from the metabolism of fatty acids, are produced in the liver are released into the bloodstream, so that they may travel to and be used by brain mitochondria to produce ATP. Astrocytes are capable of synthesizing and releasing ketones as well. There is no evidence currently to suggest that neurons can synthesize ketones. It is also not clear what proportion of ketones used by the brain are produced peripherally in the liver or centrally in the brain. Ketones produce about 22 units of ATP per molecule.
Lactate can be used as a fuel in both the brain and the body. Lactate is produced from pyruvate (see Figure 1.), a product of glycolysis that will mostly enter the mitochondria and eventually produce ATP. That which does not enter the mitochondria is converted into lactate in the cell by an enzyme called lactate dehydrogenase and then recycled back into pyruvate to eventually be used by the mitochondria to make ATP. This is if all is going well. With inadequate thiamine or extreme stress, both lactate and pyruvate build up in the cell and energy production in the mitochondria wanes.
Do Neurons Use Amino Acids From Protein for ATP?
Yes, it appears they do. Research over the last few decades has shown that, like the mitochondria in the body, those in the brain also use amino acids from protein to produce ATP. Like the body, however, there are regional/cell type and situational preferences. In general, the astrocytes appear to be more adept metabolizing different fuels into ATP than the neurons, but this may be related more to differences in experimental design than actual function. That said, there is a small but growing body of research indicating that some neurons use amino acids to produce ATP.
How the brain derives ATP from the amino acids is a bit complicated and probably requires its own article. Briefly, however, there are two main entry points into the mitochondria: the branched chain amino acid (BCAA) pathway, which catabolizes valine, leucine, and isoleucine (upper left of Figure 1.) or via the enzyme alpha-ketoglutarate dehydrogenase (aKGD – lower right of Figure 1.), which takes glutamate and feeds it through the TCA. In peripheral mitochondria, BCAA pathway yields differing amounts of ATP depending upon the specific amino acid and the path it takes, while the aKGD pathway may produce up to 24-27 ATP.
One study, using cortical neurons in a culture medium, found that glutamate could be used to produce ATP when glucose was low. Here, when researchers prevented pyruvate from entering the mitochondria (top pathway in Figure 1.), glutamate entry via the aKDH enzyme was upregulated so that ATP production could be maintained. The aKGD is a common in peripheral cells, particularly in cancer and immune cells where replication is prioritized. It had not been observed previously in brain cells (that I am aware). In the brain, glutamate is viewed as an excitatory neurotransmitter, one that can become excitotoxic to the neuron when concentrations become too high. This study showed that it may be also involved with ATP synthesis, and as such, it is one of the mechanisms by which mitochondria protect neurons from glutamatergic insults. In another study, where severe hypoglycemia was induced in rodents and brain slices reviewed, researchers found that glutamate metabolism via oxaloacetate pathway (upper left of TCA cycle in Figure 1.) was upregulated in neurons and fed through the TCA cycle to maintain ATP production. In astrocytes, this pathway may produce 9-12 molecules of ATP.
Serine and glycine (not shown and deserving of a more in depth discussion, along with all of the other conditionally essential and non-essential amino acids) are important intermediate metabolites contributing indirectly to glycolysis and eventually the TCA cycle. In cancer cells, one molecule of glycine imported into the mitochondria will yield one unit of ATP. It is not clear how many units of ATP are produced from amino acids in neural mitochondria, but since neurons and glial cells contain much, if not all, of the same metabolic machinery, it is not unwarranted to think that these cells would not be just as capable of producing quantities of ATP similar to that in cells of the body.
Finally, some years ago, researchers found that the orexin/hypocretin neurons (same neurons, different names) responded to amino acids by firing, while effectively shutting down in presence of glucose. Fatty acids had no effect. The orexin/hypocretin neurons are considered energy sensors for the brain and the body, and as such, they regulate arousal and wakefulness, appetite, emotion, pain response, and a whole bunch of other stuff. The nuclei of these neurons are located in the lateral hypothalamus and have vast projections throughout the brain with receptors all over the body. Researchers ranked the amino acids that induced firing in order of potency: glycine > aspartate > cysteine > alanine > serine > asparagine > proline > glutamine. Of interest, when the orexin/hypocretin neurons stop or slow firing, the physiological response is increased sleepiness (these neurons have to fire to maintain wakefulness), decreased appetite (anorexia), and increased pain sensitivity (the release of endogenous opioids is reduced). This appears to be the seat of what we might call the stress or sickness response.
These are just a few of the papers published in recent years illustrating the use of proteins in neurons and glial cells. While certainly not dominant pathways of ATP synthesis, the contributions are not inconsequential.
Fatty Acids For Brain Fuel
Although ketones derived from fatty metabolism have long be considered integral to brain energetic metabolism, the fatty acids themselves have been largely disregarded, at least with regard to ATP production in neurons and in the adult brain. Developmentally, fatty acid oxidation is a key energy pathway. In the adult brain, however, glucose dogma persists. That said, fatty acid metabolism in astrocytes has been documented for a few decades now and is estimated to account for at least 20% of the total brain ATP pool. Much of the research involving neurons, however, remains equivocal.
I believe that contradictions observed in this arena are due largely to errant assumptions and design decisions. Recall, that in the body there are regional and cell-type differences in preferred fuel substrates. Not all cells use all fuels equally. I suspect the brain is no different. In that regard, any investigation into fuel preference of neurons must be painstakingly specific, and more than a little bit creative. For this reason, mixed slices of brain tissue, a typical medium for this type of work, is likely to yield spurious results. Similarly, non-physiologically intact tissue measuring acute uptake of fatty acids, disregards the metabolic time course and complement substrates needed compared to glucose. Finally, like with other cells, the mitochondria within neurons, never metabolize a single substrate in isolation or metabolize the same pattern and proportion of substrates at rest as during activity. Isolated measures at rest, therefore miss the larger patterns. There are other problems, of course, but these issues constrain what can be identified under the current paradigm.
That said, there are ways to identify fatty acid metabolism in neurons. One particularly insightful study published just this month, did so by blocking a gene linked to a disease called hereditary spastic paraplegia (HSP). HSP is a neurodegenerative disorder associated with degradation of the neurons in the cortico-spinal tract and in other regions of the central nervous system (CNS). Key symptoms include muscle spasticity and intellectual disability. The gene in question, DDHD2, is involved in lipophagy in the CNS. When it is blocked, lipids accumulate in the synaptic terminals of neurons and fatty acid flux between the neuron and its mitochondria diminish, spurring a loss of mitochondrial membrane integrity and a reduction of energetic capacity. The resulting process and symptoms indicate, at least indirectly, that fatty acids are needed for mitochondrial energetics in these neurons.
Another study looked at energy substrates for hippocampal stem/progenitor cells (hippocampal neurons are responsible for memory consolidation) and found that a shift from glucose to fatty acid metabolism regulates stem cell proliferation in adult rodent brains, which in turn indicates fatty acids are required for learning. A study using fruit flies confirmed that fatty acid metabolism was required in hippocampal neurons during ‘intense learning.’ Their absence reduced memory formation.
Using a rodent model of post-traumatic stress disorder (PTSD), researchers observed reduced fatty acid oxidation and impaired mitochondrial function in the cerebellum in PTSD versus control animals. The cerebellum is the area of the brain responsible for coordinating balance, movement, and posture, but is also related to maintaining control of executive type functions and emotional tenor.
Research in the Parkinson’s arena suggests that the dopaminergic neurons in the substantial nigra require more ATP and flux through OXPHOS than other neurons. With that there are hints peppered throughout the literature indicating that aberrant fatty acid metabolism may be a component of Parkinson’s related energy deficits in these neurons, but unfortunately I could find no direct evidence.
From the aforementioned studies, it appears that the brain does, indeed, use fatty acids to make ATP. With the paucity of research and the limitations of study design, however, it is difficult to ascertain what percentage of ATP in neurons is metabolized via fatty acids. We know that at least 20% of ATP in astrocytes comes from one medium chain fatty acid, but that is about it. From various studies involving neurons in specific brain regions, it appears that during certain situations, fatty acids are favored over glucose. Specifically, when neurons are firing, fatty acids may become a more dominant fuel source. Accordingly, from one of the researchers who identified lipid use in the cortico-spinal neurons:
‘The process of being able to use the fat is controlled by the electrical activity of the neurons, and I was shocked by this finding,’ Dr. Ryan said. ‘If the neuron is busy, it drives this consumption. If it’s at rest, the process isn’t happening.’
Given that the brain requires such an immense amount of energy to function and survive – approximately 5.7kg per day or 5x its weight, I have to believe that it makes sense to use all available energy producing substrates, not just the easiest and quickest. The fact that we have not found more evidence seems to be more of a problem of methods than anything else. When we get the right methods, I believe it will become clear that neurons use fatty acids just like every other cell in the body.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
The feature image was created using Canva AI.













