I regularly receive correspondence from people asking whether thiamine tetrahydrofurfuryl disulfide (abbreviated TTFD), a thiamine (vitamin B1) derivative, is toxic or not. Most people following this line of inquiry base the assumptions of “toxicity” on statements previously made by the famous (and now deceased) Andrew Cutler, PhD. Cutler is most well-known for his work on mercury chelation and detoxification protocols and has amassed thousands of followers over the years. He was strongly opposed to the application of TTFD therapeutically and explicitly advised people against using this molecule as a nutritional supplement for thiamine repletion or heavy metal detoxification. Much of what he said on this topic was documented in the archives of the Onibasu website which can be found here. Cutler’s statements were speculative in nature, based on anecdotes, and to my knowledge, were never backed by any scientific evidence. In this article, I would like to address his claim that TTFD is toxic. I should note, based upon all of the available research, which is substantial, TTFD is not toxic, even at supra-physiological dosages. A version of this article was published on my website.
Is TTFD Toxic?
To understand whether TTFD or any compound may be considered toxic, it is important to recognize how toxicity is determined in pharmacology. Here toxicity is represented by something called a therapeutic index (TI). In animals, where much of the research is conducted initially, the TI is determined between by ratio of the lethal dose 50 (LD50), a dose at which 50% of the animals die, and the effective dose 50 (ED50), the dose at which a therapeutic response is noticed in 50% of the animals. This is represented as TI = LD50/ED50. In humans, instead of lethal dose, toxic dose is used (TD50). Here, the TD50 represents negative or adverse reactions in 50% of the population tested. The equation is basically the same, TI = TD50/ED50.
In both cases, higher therapeutic dosing windows relative to efficacy confers greater drug safety. Compounds where there is little wiggle room between the TD50 and LD50 – a small TI – are considered the most toxic. See Figure 1.
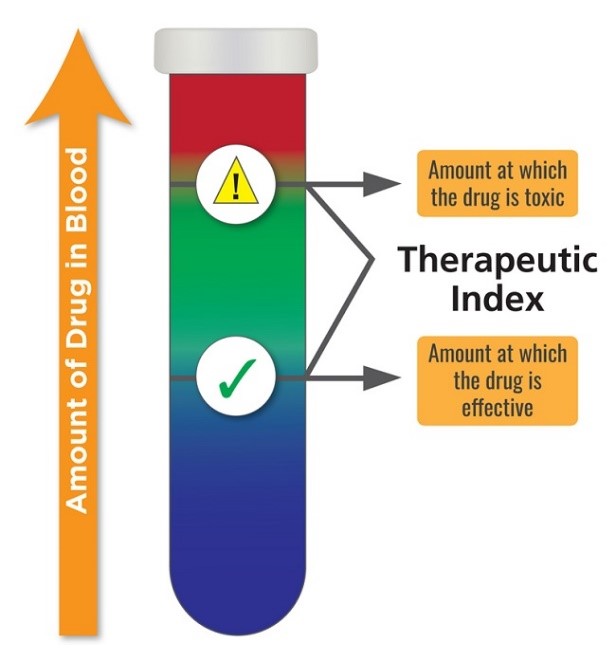
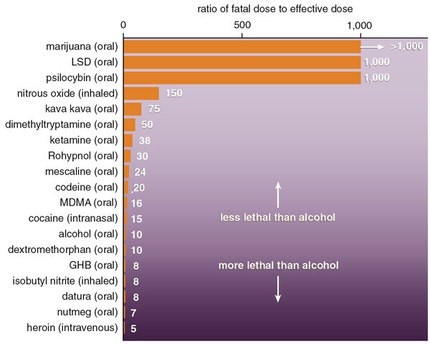
By way of example, alcohol/ethanol has a TI of 10, whereas LSD and cannabis have TI’s of greater than 1000. This means that one has to consume far less alcohol to achieve toxicity and lethality than either LSD or cannabis. Indeed, it is virtually impossible to overdose (OD) with LSD or marijuana compared to the ease at which one can OD on alcohol. Figure 2.
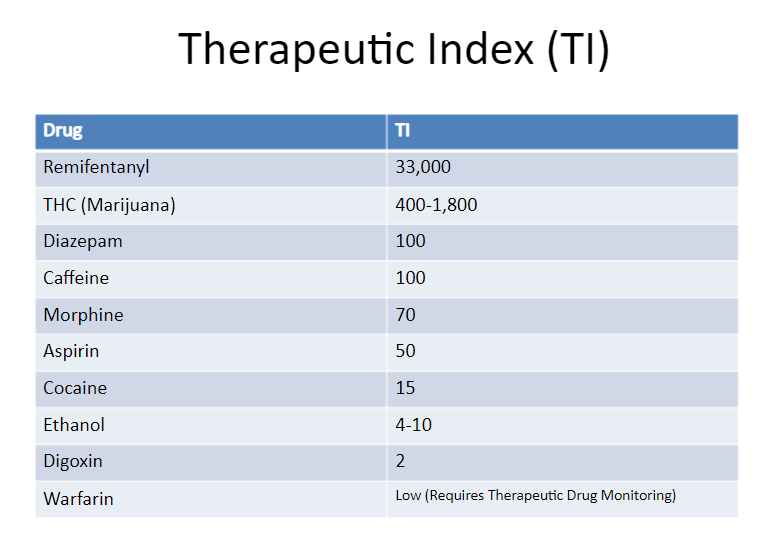
Similarly, the TI for many common drugs is quite low. Figure 3. from a pharmacology lecture on SlideShare, shows the TI of common medications.
What Does Any of This Mean for TTFD?
In mice studies, the LD50 for TTFD has been calculated at 450mg/kg when given intravenously (IV) while the LD50 for an oral dose is 2200mg/kg. Since the conversion from mice to human dosage is not calculated directly based upon on weight, but accounts for interspecies differences in metabolism, an equivalent LD50 for a 70 kg (154 lb) human would be 2.5 grams of TTFD when administered via IV and 12.5 grams if taken orally. Whilst 2.5 (IV)-12.5 (oral) grams may seem low, no one realistically takes that amount of TTFD per day for therapeutic reasons. The highest oral doses I have observed are around ~1-2 grams per day. These are individuals with chronic thiamine deficiency, usually accompanied by underlying genetic issues. More commonly, observation and clinical research suggest between 100-300 mg/day is used for most treatment/research protocols (see the ‘What about Research in Humans’ section below).
Back to the math, the RDA for thiamine, not TTFD, but thiamine consumed from food naturally, from food fortification and/or via supplementation using the most common formulations of thiamine mononitrate or hydrochloride (HCL) is 1.1 and 1.2 mg per day for adult females and males respectively. Assuming that the RDA values represent an effective dose (there are no actual data on the ED50 for thiamine), and for simplicity, that TTFD is as effective as the other two formulations, when we calculate the TI for humans (TI= ED50/TD50) for humans, we get a huge range between effective dose and toxic or lethal dose. For IV administered TTFD the ratio would be ~2,500:1, while oral dosing, it would be ~12,500: 1. If we assume, based upon its bioactivity that TTFD is more potent than the other two formulations, the TI would increase even more.
Based just upon standard toxicology parameters, it is clear that TTFD is not toxic. An individual would have to take ~12,000 times the effective dose to approximate lethality. I say approximate, because there are no documented cases of TTFD overdose. Lethality, however, is just one component of toxicology. Adverse reactions are an important consideration. That is why, in human research, instead of LD50, TD50 is used. Here though, the work becomes a little murky, partly because animals are used and partly because the chemistry of TTFD metabolism is complicated.
Toxicity Studies Using High Dose TTFD
Consistently, the animal data show that excessive doses for extended periods of time, even during pregnancy and across multiple generations, TTFD is not toxic.
- Research performed on the reproductive effects of TTFD in monkeys showed that massive doses of 500mg/kg, close to supposed LD50 for that species, found no deaths. To put this in context, it would be the human equivalent of taking 10-11 grams per day for MONTHS.
- That same study also looked at massive doses in rabbits and found no significant increase in incidence of fetal malformations in either group was observed, even in groups treated with high doses and no significant teratogenic effects or developmental abnormalities in pregnancy occurred.
- As referenced in this document, Takeda’s research by Mizutani demonstrated that administration of 100, 300 and 500mg/kg in rats for two generations from the time of maturation to the time of reproduction showed no abnormalities. The average human equivalent (70Kg) of these doses would be 570mg, 1.7 grams and 2.8 grams per day for life.
- The results of another study showed that long-term oral administration of 30-300mg/kg to pregnant animals failed to produce any significant developmental abnormality. Intraperitoneal administration of 1000mg/kg also showed no sign of chromosome aberration, damage to sex organs or spermatogenesis.
TTFD Metabolism
If TTFD is not toxic via the traditional measures, is there something about the molecule itself that may be problematic and cause unwanted effects? Cutler speculated that the mercaptan part of TTFD was responsible for toxicity, and that this primarily affected the liver. The word “mercaptan” refers to the thiol group that breaks away from thiamine after its absorption into the cell. This mercaptan group essentially accounts for the “TFD” of the abbreviation TTFD. After TTFD is absorbed, it gets “broken apart” (the disulfide bond is chemically reduced) by glutathione, cysteine, or hemoglobin to release the free thiamine molecule, which will become trapped inside the cell and ultimately used by the body.
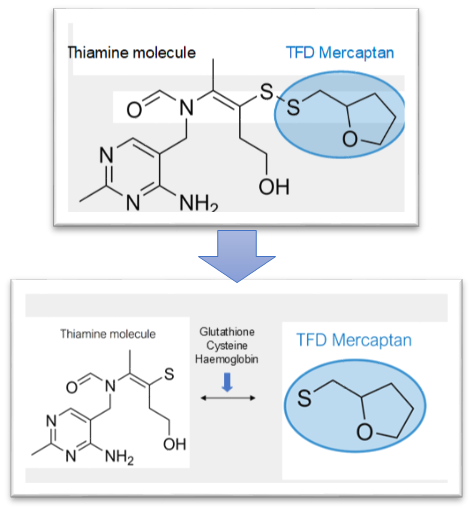
In more technical terms, the prosthetic mercaptan is released and then rapidly metabolized by the liver through methylation and later sulfoxidation by liver mono-oxygenase enzymes into breakdown products which are then excreted in urine. Is mercaptan toxic as Cutler suggested? The original series of studies on the enzymatic breakdown of TTFD and mercaptan show that it is not toxic and is rapidly excreted from urine.
- The metabolic fate of methyl tetrahydrofurfuryl sulfide and its related compounds in rats
- Enzymatic Studies on the Metabolism of the Tetrahydrofurfuryl Mercaptan Moiety of Thiamine Tetrahydrofurfuryl Disulfide Microsomal S-Transmethylase
- Enzymatic Studies on the Metabolism of the Tetrahydrofurfuryl Mercaptan Moiety of Thiamine Tetrahydrofurfuryl Disulfide: II. Sulfide and Sulfoxide Oxygenases in Microsomes
- Enzymatic Studies on the Metabolism of the Tetrahydrofurfuryl Mercaptan Moiety of Thiamine Tetrahydrofurfuryl Disulfide: III. Oxidative Cleavage of the Tetrahydrofuran Moiety
- Enzymatic Studies on the Metabolism of the Tetrahydrofurfuryl Mercaptan Moiety of Thiamine Tetrahydrofurfuryl Disulfide: IV. Induction of Microsomal S-Transmethylase, and Sulfide and Sulfoxide Oxygenases in the Drug-treated Rat
If mercaptan itself is not toxic, perhaps it metabolizes into something else and one of its by-products are problematic. From Figure 5., we see that mercaptan is metabolized into a sulfate that is then eliminated via urinary excretion and a few other compounds that are processed by the liver first before being eliminated via urinary excretion as well. The breakdown products are shown below in Figure 5.
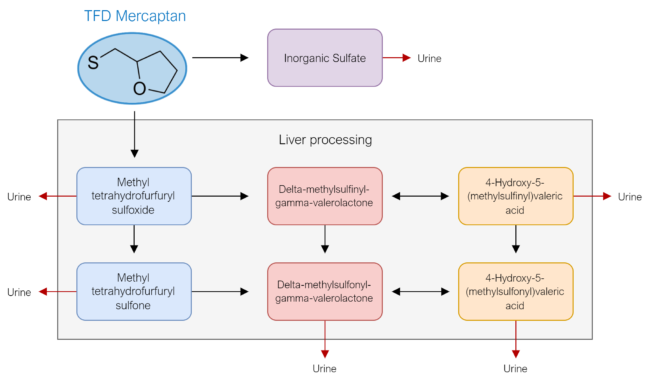
A study titled “Pharmacological study of S-alkyl side chain metabolites of thiamine alkyl disulfides” sought to determine the acute and sub-acute toxicity levels of each metabolite. They concluded that toxicity of these breakdown products was low. Remember the LD50, the dose that causes death in 50% of the study animals, research shows that the LD50 each of these breakdown products is enormous, far more than would be clinical relevant in humans. The results of this study showed:
- Inorganic sulfate: non-toxic
- Delta-methylsulfonyl-gamma-valerolactone: also non-toxic.
- Intravenously, the LD50 in mice was in excess of 5 grams/kg body weight. For comparison, the LD50 estimate for a 70KG human: 5 grams intravenously.
- Orally the LD50 in mice was 6 grams/kg body weight, which, for a 70 kg human would translate to approximately.
- 4-Hydroxy-5-(methylsulfonyl) valeric acid
- Intravenous LD50 in mice: 1.5 grams/kg body weight
- LD50 estimate for a 70KG human: 3 grams intravenously
Once again, it appears that none of these compounds is toxic. In humans, approximately 82-90% of these metabolites are excreted within 24hrs and 100% are excreted within 48hrs. What this tells us is that by themselves, these metabolites are not toxic except in supra-physiologic doses, which are not relevant from a clinical perspective.
What About Liver Damage?
Cutler speculated that metabolism of TTFD resulted in liver damage. To quote Cutler:
My guess is the tetrahydrofurfuryl mercaptan part kills your liver.
Here too, however, the data suggest otherwise. In animals with artificially induced liver damage by carbon tetrachloride and/or hepatic dysfunction due to choline deficiency, the breakdown products of TTFD were assessed. They showed that the quantity of excreted metabolites in the hepatotoxic group were equal to the control, and in choline deficiency the quantity of excreted metabolites was only slightly reduced. In the hepatotoxic group, a qualitative difference was found with a lower proportion of methyl metabolites (MTHFSO, MTHFS02). This suggests, even in with pre-existing or induced hepatotoxicity, TTFD can be excreted albeit slightly differently.
A peer-reviewed study published in 2018 entitled “The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement” studied the effects of different doses of TTFD in 30 animals for a period of 6 weeks. The highest oral dose used was 500mg/kg in 10 test subjects, which is the human equivalent to 40mg/kg which, in a 70KG human, is 2.8 GRAMS per day for 6 weeks. Remarkably, they showed that this highest dose produced significant improvement in endurance capacity and lactate homeostasis.
More importantly, this study also performed comprehensive measures of sub-acute toxicity with the aim of evaluating the safety of high doses in humans. Even at the highest dose taken for 6 whole weeks, no changes in behavior, diet, growth curve, or organ weight (liver, kidney, muscle, heart, lung etc.) was observed. Furthermore, to assess liver function, they performed comprehensive metabolic analysis including liver enzymes (ALT, AST), creatine, uric acid, total cholesterol, triglycerides, albumin, total protein, ammonia, creatine kinase, and total protein. The only significant changes were a slight reduction in total cholesterol and significant reduction in lactate, creatine kinase and blood urea nitrogen (all of which are considered positive changes). Every other liver marker was perfectly in range. To gain further insight into the liver function and the health of other tissues, they performed histopathological analysis of the tissue under microscope and showed that massive doses caused no pathological changes in any tissue whatsoever. The authors conclude:
In the current study, we proposed that the higher thiamine derivative, TTFD, could significantly improve physical activities and physiological adaption with evidence-based safety validation. For practical application, we recommend that athletes should consume a daily intake of 40 mg/kg TTFD (equivalently converted from mouse 500 mg/kg dose based on body surface area between mice and humans by formula from the US Food and Drug Administration [36]) to improve energy regulation for higher performance in a combined nutritional strategy, including carbohydrate loading for efficient energy demand during extended exercise.
They were so convinced of the supplement’s safety that they recommended athletes take the equivalent of 2.8 GRAMS per day LONG-TERM to improve athletic performance.
What About Research in Humans?
To those who complain that these are “animal studies”, the comparative metabolic studies have found that the metabolism of TTFD is essentially the same in animals and humans. This means that humans are likely to respond similarly. As one of the first medical doctors to use TTFD as a clinical intervention in the Western world, Dr. Derrick Lonsdale obtained a special license from the FDA to import this molecule and studied its effects in his pediatric patients. In his own words:
I was able to study the value of this incredible substance in literally hundreds, if not thousands of patients. Far from being toxic, as this person claims, I never saw a single item that suggested toxicity.
Some reports published by Lonsdale and other authors include:
- 22 children with Down’s Syndrome, 12 of which were administered TTFD for 12 months and 12 of which were administered TTFD for 6 months. No serious adverse events noted.
- Brainstem dysfunction – three infants saw symptomatic improvement with thiamine disulfide treatment.
- Abnormal brainstem auditory evoked potentials, one infant was administered intravenous TTFD and displayed normalization of brainstem function
- 21 patients subacute necrotizing encephalomyelopathy treated with thiamine derivatives TPD/TTFD 10 Children – no serious adverse events – 1 experienced worsening of behavior/symptoms, 2 with rash
- 44 polyneuropathy patients treated with 50mg TTFD injection, no adverse effects reported.
- Prosultiamine (TPD) at 300mg per day for 12 weeks (TPD, a very similar molecule to TTFD) used to treat spinal cord injury in human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis (2013). Significant improvement in motor functions and bladder control, as well as reducing viral numbers in blood. Only adverse symptom was mild epigastric discomfort. No safety concerns.
For a comprehensive look at thiamine research, refer to Drs. Lonsdale and Marrs’ book: Thiamine Deficiency Disease, Dysautonomia and High Calorie Malnutrition
TTFD Used in Other Countries
It is worth noting that TTFD is not well known in Western medicine. The regions of the world in which it is used extensively include Japan, China, and other countries in the Far East.
Japanese Cases
Unfortunately, much of the Japanese literature is not published in English, so it can be difficult to find. I use a translator application called DeepL to read these articles. Furthermore, TTFD is so regularly prescribed for treating thiamine deficiency that much of the literature refers to TTFD simply as “thiamine” or “vitamin B1”, using the terms interchangeably. This means that finding research papers without TTFD in the actual title is very difficult. Below are 33 case reports from the Japanese literature, including some in children, which document the benefits of TTFD clinically. In all of the papers I have read, I have not once seen mention of safety concerns using this. In several reports, hundreds of milligrams are maintained indefinitely with no apparent issues.
- 267 cases of sudden-onset deafness was treated with 150mg TTFD orally, with most therapeutic effect seen after 2-3 months of treatment
- 20 cases of perceptive deafness, 20 cases of laryngeal disease, 7 cases of facial nerve palsy and 3 cases of anosmia injected with TTFD 50mg once per day for 5-20 days. 60% effective and no side effects.
- 24 subjects without nutritional deficiency, 20 cases of alcoholism, and 48 cases of alcoholism with signs of deficiency and/or liver disease were given either TTFD, Thiamine propyldisulfide (a similar disulfide derivative), or thiamine HCL. They showed no toxic effects at 3-6 months in any group, and demonstrated that oral TTFD/TPD increased whole blood, erythrocyte, and cerebrospinal fluid thiamine levels at an equivalent level to intravenous thiamine HCL.
- Beriberi treated with 150mg IV one week, followed by 100mg oral long-term (2014)
- Cardiac failure 100mg IV (1987)
- Heart and circulatory failure (2008)
- TTFD administered to 15 year old boy to treat beriberi, remained on the therapy long-term
- 18 patients with non-diabetic peripheral neuropathy 1-3 months, no serious side effects
- Wernicke encephalopathy in hemodialysis – 100mg/iv, later oral continued for 2 months until improvement (2009)
- Diabetic lactic acidosis – 100mg/day IV for 7 days resolution in symptoms, followed by 75mg indefinitely (2008)
- Beriberi w/ pulmonary hypotension – 50mg long-term (2019)
- E/beriberi after intestinal resection – 150mg IV three days, 75mg long-term (2018):
- E/Shoshin beriberi – 150mg/IV, 75mg oral long-term (2013)
- A case of Wernicke’s encephalopathy with severe cardiac sympathetic dysfunction – 100mg (2012)
- Marked anasarca with impaired consciousness, which was thought to be caused by shoshin beriberi due to impaired vitamin B1 utilization. – TTFD 40mg +400mg HCL, followed by 2 months+ TTFD 100mg (2015)
- Beriberi neuropathy and shoshin beriberi that developed 6 years after gastrectomy on the cardia side – 100mg TTFD long term (2013)
- Chemotherapy induced W.E – IV thiamine HCL followed by TTFD 75mg long-term (2008)
- Postoperative W.E treated with 100mg IV TTFD (1998)
- Cardiomyopathy associated with mitochondrial disease that developed heart failure, treated with 100mg long-term (2017)
- Mitochondria rescue formula recommended for acute encephalopathy: including TTFD 100mg (2019)
- Pediatric acute encephalopathy neuroprotection protocol – cocktail including 200mg TTFD in 15 children (2013)
- 200mg TTFD x 31 children childhood acute encephalopathy (part of protocol) (2014)
- Lactic acidosis caused by low-dose metformin: Thiamine HCL 100mg followed by 75mg TTFD long-term, normalization of all liver values (2014)
- Beriberi mimicking Guillain-Barré syndrome – IV TTFD 100mg resolved this
- Shoshin beriberi – IV TTFD 150mg for 11 days, indefinite oral dose 150mg TTFD long-term
- 6 infants (0-1 yrs old) treated with TTFD for childhood congenital lactic acidosis. Doses included 35mg/KG – 50mg/KG. Some cases were unresponsive to thiamine HCL, where TTFD ONLY could reduce lactate significantly “Fursulthiamine hydrochloride was significantly superior to thiamine hydrochloride in reducing lactate.” Only the cases which used TTFD survived. Children were kept on high doses permanently with no adverse effects.
- 75mg TTFD improved cerebral blood flow in deficiency
- 75mg TTFD used in mitochondrial myopathy long-term
- 50mg TTFD used to treat edema and weight gain and marginal thiamine deficiency – authors recommend TTFD instead of thiamine HCL (2021)
- Biguanide-induced lactic acidosis treated with 100mg, then 300mg TTFD (2017)
- 100mg used to treat encephalopathy w/ hyperammonemia (2003)
- Subacute spinal degeneration caused by B12 deficiency treated with B12 and 75mg TTFD long-term (2020)
- Statement by a Japanese physician: recommendation to use TTFD instead of thiamine HCL due to superior qualities.
Chinese Cases
Like the Japanese research, most (if not all) of the Chinese studies using TTFD are not published in English. However, it is clear that the Chinese medical system uses TTFD frequently and has done so for several decades. Most of the studies below were reported within the last 20-30 years. Once again, I could not find any concern regarding the safety of this molecule and it was demonstrated as remarkably effective for a variety of conditions. Interestingly, the Chinese not only use it for deficiency, but also for non-deficient conditions where it is often injected directly into acupoints (acupuncture meridians) either alone, or in combination with other nutrients/medications. They still use these methods to this day. Here are 32 more articles regarding the safety and efficacy of TTFD from the Chinese literature.
- 194 cases of infantile beriberi cured with IM/IV thiamine and TTFD (1987)
- 50 infants treated with TTFD for cardiac beriberi (1997)
- 70 children with infantile beriberi cured with intravenous TTFD (1990)
- 48 cases of infantile cerebral beriberi (0-3 years old) treated with TTFD (1997)
- 35 cases of infantile beriberi cured TTFD (2010)
- 10 cases of infantile cerebral beriberi cured with B1 HCL and TTFD
- 10 cases of cerebral beriberi and basal ganglia damage treated with TTFD injections (2003)
- 125 children with pneumonia treated using TTFD as primary treatment (10mg IM <3 months old, 20mg IM <6 months, 20mg twice per day >6 months old)
- 283 out of 285 children with rectal prolapse cured by TTFD injection into “changqiang” acupoint (1988)
- 89 cases of rectal prolapse also treated with TTFD acupoint injection (1998)
- 50 cases of cerebral hypoplasia improved with acupoint injection of acetyl glutamine and TTFD (1983)
- 35 patients treated for hyperthyroidism with TTFD as adjunctive treatment (1999)
- 50 cases of costochondritis cured with Analgin + TTFD injection (1993)
- 13 children with ocular nerve palsy cured with TTFD (2010)
- 50 cases of urinary incontinence treated with acupoint injection of combination of acetyl glutamine, TTFD and/or r-aminobutyric acid (1990)
- 26 cases of delayed peripheral neuropathy due to organophosphate poisoning treated with acupuncture and TTFD injection (2001)
- 47 cases of lumbar disc protrusion treated with acupoint injection, B12 and TTFD (1994)
- 38 cases of facial neuritis treated with acupuncture and vitamins including TTFD injection (1999)
- 60 cases of migraine treated with Chinese medicine, flunarizine, and TTFD (2004)
- 24 cases of migraine treated with TTFD acupoint injection (1990)
- 40 patients with cerebrovascular disease addressed using acetyl glutamine and TTFD scalp acupoint injections (2001)
- 30 cases diabetic neuropathy, 75mg TTFD used as a control in– 60% effective (2002)
- 69 cases of Bell’s Palsy, TTFD used with acyclovir (1999)
- 120 cases of Bell’s Palsy treated with oral TTFD, methylb12, and/or electroacupuncture and facial muscle exercise (2019)
- 65 cases of Meniere’s Diseases treated with TCM, vitamins including TTFD injections
- 118 cases of herpes zoster treated with TTFD in conjunction with acyclovir and traditional Chinese medicine (2013).
- 100 cases of senile deafness treated with cocktail including TTFD (2000)
- 36 cases of cervical spondylotic radiculopathy treated with control of TTFD and naproxen – 75% effective (2009)
- 60 cases of postherpetic neuralgia treated with cocktail including TTFD
- 1 case of polerarteritis nodosa w/peripheral neuritis treated with cocktail including TTFD
- 1 case of central paralytic dysphagia (tuberculosis meningitis) unresponsive to conventional treatment cured by injection of TTFD at meridian acupoint (1974)
- 1 case of drug-induced diplopia treated with methyl B12 and TTFD
TTFD Is Not Toxic
At this point, it should be clear that TTFD is not toxic at either therapeutic or even supraphyisiological doses. This is supported by in vitro, animal, and human studies. One would have to use ~12,000 times the therapeutic dose to approximate toxicity and even then it is not clear that there would be the problems that Cutler suggested. That is not to say that everyone who takes this supplement responds favorably. Clinically, there are individuals for whom other formulations of thiamine work better. This is generally related to a lack of the necessary nutrient cofactors involved with the detox enzyme glutathione. I have written about that previously here. That perceived intolerance, however, is not the same as toxicity. The toxicity data are clear. TTFD is safe. The clinical data are also clear. TTFD is effective. The molecule has been in use for over half a century and is used extensively in medical practice in Eastern countries. No safety concerns or claims of toxicity have been raised, apart from those made by Cutler.
We Need Your Help
More people than ever are reading Hormones Matter, a testament to the need for independent voices in health and medicine. We are not funded and accept limited advertising. Unlike many health sites, we don’t force you to purchase a subscription. We believe health information should be open to all. If you read Hormones Matter, like it, please help support it. Contribute now.
Yes, I would like to support Hormones Matter.
Photo by Matt Walsh on Unsplash.
This articles was published originally in February 2022.














The thiols could cause reductive stress.
This would depend on the individual’s state when they first start supplementation.
Or they may get there with time or increased doses.
Or they pair it with too many anti-oxidants, etc.
This may have something to do with people who cannot tolerate TTFD, or whom after some time, things go south / back to how they were before.
I think this is what that person is referring to me as sulfur ‘intolerance’.
I wouldn’t be surprised if said intolerance is aleatory.
If a person has a sulphur intolerance – genetic, would TTFd still be the better form to take?
I have the same question.
Symptoms return if the “ideal dose” is exceeded. The required action is to reduce the dose. Try to find the ideal dose by trial and error. It is a completely new treatment method and will require further clinical research.
After taking 100mg of TTFD/per day for about a month, I started seeing a flash of light in the corner of my eye. This happened multiple times a day. I’ve seen some people call it a type of floater.
After some research, some folks attribute this to a B12 deficiency. And, isn’t B1 an antagonist of B12?
So, I switched to the HCL version and the flash has slowly decreased almost down to zero. That is until I took 500mg of HCL one day. The flash can back temporarily.
Have you heard of this before?
I’ve been taking 600mg of HCL for 3 weeks and although I think it’s been helping at first, now I’m having trouble breathing everyday and I feel so tired. Is it paradoxical reaction ? I think it’s weird that the worsening happened after 2 weeks, I was doing fine before that. I want to continue taking thiamine if it can help me but I’m worried of making my state worse, or that it could even be dangerous. (having troubles breathing is scrary !)
I’m a 34 yo male who has chronic fatigue syndrome btw. I’m taking potassium and magnesium at the same time.
Thanks a lot in advance if anyone can reply.
Something to consider:
I see this often played out in Me/CFS:
An individual will try x substance, it gives benefit. They then start to do more, pushing past their energy envelope and will inevitably crash. Because they are not aware of this push and crash dynamic, they think it is the X substance.
I think one benefits from pairing all modalities, B1 + keeping to your current baseline of energy generating capacity. With time, it expands and you can do more. Keep in mind, the brain needs and uses A LOT of energy. To sleep, your brains need energy. To relax your muscles, they need energy.
Something else to consider:
Are you eating enough? You need substrate to make energy from. B1 let’s your body use more appropriate process of making energy, and your feeding needs might change. Perhaps you were undereating before too, digestive problems etc.
What to eat? A good rule of thumb is to follow cravings. Better than imposing a belief system, such as keto, paleo, low fat, low carb, etc.
Another thing to think about:
It’s funny how the medical/food industry labels sugar/salt/fat as dangerous to your health, and keeps quiet on the additives and chemical industrial substances added to all kinds of foods. Even flour and milk, basic foods
Godspeed!
where is the sulfur ? article got realesed you couldnt wait to give ancedotal inference?
where do you think is taken TTFD out? thin air?
TTFD is taken from garlic bulb…garlic is naturally high in sulfur…
https://www.livestrong.com/article/239140-what-vitamins-contain-sulfur/
I want to share my experience, I took ttfdaround 5/6 months until weird symptoms began to appear such as insomnia, diarrhea and joint pain. I didn’t know what was happening and I thought I had crohn’s or something similar. I did a colonoscopy and they found ibd but no signs of disease. So my doctor suggested some food intolerance…thus began the journey of trial and error…until after several months since my symptoms started I found the culprit “Sulfur Intolerance”…yes, TTFD caused an excess of sulfur in my body, which was resolved with a low sulfur diet along with a lot of liquid molybdenum, vitamin C and manganese for several weeks…and of course a lot of time and money invested in the whole process…so, well, TTFD is not toxic but it could cause a lot of problems
Dr. Costatini, the Italian doctor who has successfully treated advanced Parkinson’s disease with thiamine, used as much as 4.0 grams of thiamine HCl/day by mouth. He reported his studies in a peer reviewed medical journal. His case load was 2500 with 100% success, regardless of severity. It should have set the medical profession on fire, but it hasn’t !!